Extracellular vesicle-enriched secretome of adipose-derived stem cells upregulates clusterin to alleviate doxorubicin-induced apoptosis in cardiomyocytes
- PMID: 40671119
- PMCID: PMC12265340
- DOI: 10.1186/s13062-025-00664-5
Extracellular vesicle-enriched secretome of adipose-derived stem cells upregulates clusterin to alleviate doxorubicin-induced apoptosis in cardiomyocytes
Abstract
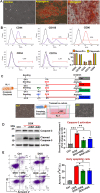
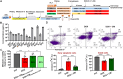
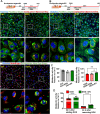
Doxorubicin (DOX) is a potent chemotherapeutic widely used against various cancers, but its clinical application is limited by DOX-induced cardiotoxicity (DIC). This study explored the cardioprotective potential of extracellular vesicle-enriched secretome derived from adipose stem cells (EVSASC) in mitigating DOX-induced apoptosis in cardiomyocytes. Adipose-derived stem cells were cultured, and their conditioned medium and extraceullular vesicles were isolated and characterized according to the Minimal Information for Studies of Extracellular Vesicles 2023 guidelines. HL-1 cardiomyocytes were pretreated with EVSASC before exposure to 1 µM DOX. Cell viability was assessed via the cell counting kit-8 assay, while apoptosis markers and survival mediators were evaluated through Western blotting. RNA sequencing identified differentially expressed genes, including clusterin (Clu), which was further quantified using an enzyme-linked immunosorbent assay. The functional role of clusterin was validated through siRNA-mediated knockdown. EVSASC significantly improved cell viability in DOX-exposed cardiomyocytes and reduced the cleaved caspase-3 to procaspase-3 ratio. Clusterin expression was highest in EVSASC-treated cells, and its knockdown markedly increased caspase-3 cleavage, confirming its pivotal role in cardioprotection. Moreover, EVSASC enhanced the phosphorylation of AKT, Bcl2-associated agonist of cell death, and glycogen synthase kinase-3β, implicating PI3K/AKT pathway activation in clusterin upregulation and anti-apoptotic effects. These findings demonstrate that EVSASC mitigates DOX-induced apoptosis in cardiomyocytes through clusterin upregulation and PI3K/AKT pathway activation. Clusterin is identified as a potential biomarker for evaluating EVSASC efficacy. While EVSASC shows promise as a cardioprotective strategy against DIC, further studies are needed to optimize its therapeutic safety by addressing potential oncogenic risks.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All animal experiments were performed following approved protocols by the Institutional Animal Care and Use Committee (IACUC) of National Taiwan University and were reported in line with the ARRIVE guidelines 2.0. (1) Title of the approved project: Mechanistic exploration of candidate oxysterols on breast tumorigenesis and tumorigenic microenvironment; (2) Name of the institutional approval committee: National Taiwan University College of Medicine and College of Public Health Institutional Animal Care and Use Committee; (3) Approval number: 20201104; (4) Date of approval: November 28, 2022. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures






Similar articles
-
Photobiomodulation Therapy Reduces Oxidative Stress and Inflammation to Alleviate the Cardiotoxic Effects of Doxorubicin in Human Stem Cell-Derived Ventricular Cardiomyocytes.Biomedicines. 2025 Jul 21;13(7):1781. doi: 10.3390/biomedicines13071781. Biomedicines. 2025. PMID: 40722850 Free PMC article.
-
Aerobic Exercise Alleviates Doxorubicin-Induced Cardiotoxicity via Inhibition of Ferroptosis.Chemotherapy. 2025;70(3):137-152. doi: 10.1159/000546096. Epub 2025 May 10. Chemotherapy. 2025. PMID: 40349681
-
LC-MS/MS based cellular pharmacokinetics to explore the mechanism of palmatine against doxorubicin-induced cardiotoxicity.J Pharm Biomed Anal. 2025 Nov 15;265:117037. doi: 10.1016/j.jpba.2025.117037. Epub 2025 Jun 19. J Pharm Biomed Anal. 2025. PMID: 40544700
-
Cardioprotective strategies against doxorubicin-induced cardiotoxicity: A review from standard therapies to emerging mitochondrial transplantation.Biomed Pharmacother. 2025 Aug;189:118315. doi: 10.1016/j.biopha.2025.118315. Epub 2025 Jul 3. Biomed Pharmacother. 2025. PMID: 40614534 Review.
-
Topotecan, pegylated liposomal doxorubicin hydrochloride and paclitaxel for second-line or subsequent treatment of advanced ovarian cancer: a systematic review and economic evaluation.Health Technol Assess. 2006 Mar;10(9):1-132. iii-iv. doi: 10.3310/hta10090. Health Technol Assess. 2006. PMID: 16545208
Cited by
-
ProDualNet: dual-target protein sequence design method based on protein language model and structure model.Brief Bioinform. 2025 Jul 2;26(4):bbaf391. doi: 10.1093/bib/bbaf391. Brief Bioinform. 2025. PMID: 40856523 Free PMC article.
References
-
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7–33. - PubMed
-
- Rivankar S. An overview of doxorubicin formulations in cancer therapy. J Cancer Res Ther. 2014;10:853–8. - PubMed
-
- Octavia Y, Tocchetti CG, Gabrielson KL, et al. Doxorubicin-induced cardiomyopathy: from molecular mechanisms to therapeutic strategies. J Mol Cell Cardiol. 2012;52:1213–25. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

