Efficacy and safety of Fe-curcumin coordination polymer nanodots to prevent corneal neovascularization in alkali burn models
- PMID: 40671122
- PMCID: PMC12265349
- DOI: 10.1186/s12951-025-03555-z
Efficacy and safety of Fe-curcumin coordination polymer nanodots to prevent corneal neovascularization in alkali burn models
Abstract
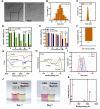
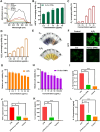
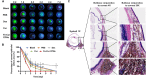
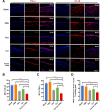
Alkali burns pose a significant risk of corneal injury, leading to potential blindness. During the progression of alkali burns, heightened oxidation levels can induce corneal damage, resulting in diminished clarity and vision loss. In this study, we chose metallic iron in conjunction with a small molecule, curcumin, to synthesize a curcumin-iron coordinated nanocomposite aimed at enhancing the bioaccessibility and targeting capabilities of curcumin. It could be found that Fe-curcumin coordination polymer nanodots (Fe-Cur CPNs) were comparably effective in suppressing corneal neovascularization, and they exhibited notable advantages in promoting corneal epithelial repair with minimal adverse effects. Additionally, Fe-Cur CPNs inhibited the activation of the nuclear factor-κ-gene binding (NF-κB) signaling pathway by scavenging reactive oxygen species (ROS), thus mitigating corneal neovascularization, which might represent a potential mechanism underlying the therapeutic effect of the Fe-Cur CPNs in alkali burn treatment. Moreover, treatment with the Fe-Cur CPNs did not result in any signs of cytotoxicity, hematological toxicity, or internal organ damage, further confirming the safety profile of this therapeutic agent. In conclusion, Fe-Cur CPNs present a novel, safe, and efficacious approach for addressing corneal alkali burns.
Keywords: Corneal alkali burn; Fe-curcumin coordination polymer nanodots (Fe-Cur CPNs); Neovascularization; Reactive oxygen species; Toxicity.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All animal experiments were conducted according to the Principles of Labora tory Animal Care of the People’s Republic of China and the Guidelines for the Care and Use of Laboratory Animals of Peking University First Hospital, China (Ethical Approval No: J202175). Consent for publication: All authors consent to publication. Competing interests: The authors declare no competing interests.
Figures



References
-
- Luo LJ, Lai JY, Chou SF, Hsueh YJ, Ma DH. Development of gelatin/ascorbic acid cryogels for potential use in corneal stromal tissue engineering. Acta Biomater. 2018;65:123–36. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

