Imatinib prevents blood-spinal cord barrier disruption by inhibiting PDGFR-mediated JMJD3 expression and activation after spinal cord injury
- PMID: 40671131
- PMCID: PMC12269138
- DOI: 10.1186/s12987-025-00690-5
Imatinib prevents blood-spinal cord barrier disruption by inhibiting PDGFR-mediated JMJD3 expression and activation after spinal cord injury
Abstract
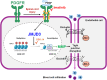
Background: After a spinal cord injury (SCI), disruption of the blood-spinal cord barrier (BSCB) leads to secondary injuries, including inflammatory responses and apoptotic cell death, ultimately causing permanent neurological deficits. Imatinib, a tyrosine kinase inhibitor, has been reported to enhance BSCB integrity and improve functional recovery after SCI. However, the mechanism by which imatinib regulates BSCB integrity remains unclear. Recent studies have identified the histone H3K27me3 demethylase JMJD3 as a key mediator of BSCB disruption, with high expression observed in blood vessels after SCI. In this study, we investigated whether imatinib regulates JMJD3 expression and activation through PDGFR signaling, thereby mitigating BSCB disruption following SCI.
Methods: Imatinib (100 mg/kg) was administered intraperitoneally to rats subjected to a contusion injury at the T9 level of the spinal cord and was continued daily for 14 days.
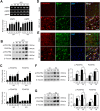
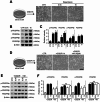
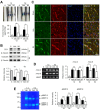
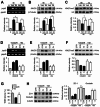
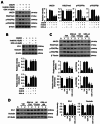
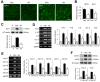
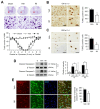
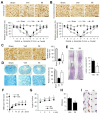
Results: Our results indicate that imatinib inhibited the phosphorylation of PDGFRα and PDGFRβ, both tyrosine kinase receptors, without affecting their expression levels. Additionally, imatinib reduced JMJD3 and MMP-9 expression and activation in blood vessels, thereby decreasing macrophage infiltration after SCI. In an oxygen-glucose deprivation (OGD)-induced bEnd.3 cell model, phosphorylated PDGFRα and PDGFRβ, along with JMJD3 expression and activation, were significantly upregulated but were effectively inhibited by imatinib treatment. Furthermore, imatinib suppressed secondary damage, including cell death, blood cell infiltration (e.g., neutrophils and macrophages), inflammation, axonal and myelin loss, and lesion volume. These effects collectively resulted in significant improvements in functional recovery after SCI.
Conclusion: Based on these findings, we propose that imatinib exerts a neuroprotective effect, in part by inhibiting PDGFR-mediated JMJD3 expression and activation following SCI.
Keywords: Blood-spinal cord barrier; Imatinib; JMJD3; MMP; PDGFR; Spinal cord injury.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All animal experiments were conducted in accordance with the guidelines set by the Animal Care Committee of Kyung Hee University (permission number: KHSASP-23-320). Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
Similar articles
-
Gallic acid attenuates blood-spinal cord barrier disruption by inhibiting Jmjd3 expression and activation after spinal cord injury.Neurobiol Dis. 2020 Nov;145:105077. doi: 10.1016/j.nbd.2020.105077. Epub 2020 Sep 6. Neurobiol Dis. 2020. PMID: 32898645
-
The beneficial effect of α-lipoic acid on spinal cord injury repair in rats is mediated through inhibition of oxidative stress: A transcriptomic analysis.J Spinal Cord Med. 2025 Sep;48(5):821-834. doi: 10.1080/10790268.2024.2342058. Epub 2024 Apr 22. J Spinal Cord Med. 2025. PMID: 38647358 Free PMC article.
-
Engineered small extracellular vesicles for targeted delivery of perlecan to stabilise the blood-spinal cord barrier after spinal cord injury.Clin Transl Med. 2025 Jun;15(6):e70381. doi: 10.1002/ctm2.70381. Clin Transl Med. 2025. PMID: 40538064 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Control of aggressive fibromatosis by treatment with imatinib mesylate. A case report and review of the literature.J Cancer Res Clin Oncol. 2007 Aug;133(8):533-8. doi: 10.1007/s00432-007-0198-9. Epub 2007 Apr 24. J Cancer Res Clin Oncol. 2007. PMID: 17453242 Free PMC article. Review.
References
-
- Jain A, Kim YT, McKeon RJ, et al. In situ gelling hydrogels for conformal repair of spinal cord defects, and local delivery of BDNF after spinal cord injury. Biomaterials. 2006;27:497–504. 10.1016/j.biomaterials.2005.07.008. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

