SIRT3 mediates CPT2 delactylation to enhance mitochondrial function and proliferation in goat granulosa cells
- PMID: 40671144
- PMCID: PMC12269156
- DOI: 10.1186/s40104-025-01231-8
SIRT3 mediates CPT2 delactylation to enhance mitochondrial function and proliferation in goat granulosa cells
Abstract
Background: Reproductive efficiency in goats is closely linked to the healthy development of follicles, with the proliferation of ovarian granulosa cells (GCs) playing a crucial role in this process. Sirtuin 3 (SIRT3), an enzyme that catalyzes post-translational modifications (PTMs) of proteins, is known to regulate a variety of mitochondrial metabolic pathways, thereby affecting cell fate. However, the specific effect of SIRT3 on the follicular development process remains unclear. Therefore, this study aimed to investigate the regulatory role of SIRT3 in the mitochondrial function and proliferation of goat GCs, as well as the underlying mechanisms involved.
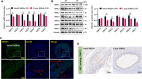
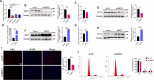
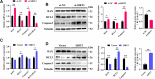
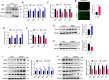
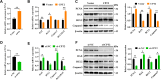
Results: In this study, GCs from small follicles in goat ovaries presented increased proliferative potential and elevated SIRT3 expression levels compared with those from large follicles. In vitro, SIRT3 overexpression enhanced mitochondrial function, promoted proliferation and inhibited apoptosis in GCs. Correspondingly, the inhibition of SIRT3 led to the opposite effects. Notably, SIRT3 interacted with carnitine palmitoyl transferase 2 (CPT2) and stabilized the CPT2 protein by mediating delactylation, which prolonged the half-life of CPT2 and prevented its degradation. Further investigation revealed that CPT2 overexpression enhanced fatty acid β-oxidation and mitochondrial function in GCs. Additionally, CPT2 promoted the proliferation of GCs by increasing the protein levels of β-catenin and its downstream target, cyclin D1 (CCND1). However, this effect was reversed by 3-TYP (a SIRT3 inhibitor).
Conclusions: SIRT3 stabilizes CPT2 protein expression through delactylation, thereby enhancing mitochondrial function and the proliferative capacity of GCs in goats. This study provides novel insights into the molecular mechanisms and regulatory pathways involved in mammalian follicular development.
Keywords: CPT2; Delactylation; Mitochondrial function; Ovarian granulosa cells; Proliferation; SIRT3.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All animal procedures used in this study were approved by the Animal Ethics Committee of Southwest University, and the approval number is IACUC-20220915-01. Consent for publication: Not applicable. Competing interests: The authors declare that they have no competing interests.
Figures






Similar articles
-
Association between mitochondrial SIRTs (SIRT3, SIRT4, and SIRT5) and PCOS.Eur J Med Res. 2025 Jul 10;30(1):611. doi: 10.1186/s40001-025-02862-3. Eur J Med Res. 2025. PMID: 40640941 Free PMC article.
-
Sirtuin 3-mediated delactylation of malic enzyme 2 disrupts redox balance and inhibits colorectal cancer growth.Cell Oncol (Dordr). 2025 Aug;48(4):979-990. doi: 10.1007/s13402-025-01058-5. Epub 2025 Apr 7. Cell Oncol (Dordr). 2025. PMID: 40192942 Free PMC article.
-
A novel effect of sulforaphane on promoting mouse granulosa cells proliferation via the NRF2-TKT pathway.J Adv Res. 2025 Aug;74:25-41. doi: 10.1016/j.jare.2024.09.020. Epub 2024 Sep 27. J Adv Res. 2025. PMID: 39341455 Free PMC article.
-
A systematic review and economic evaluation of epoetin alpha, epoetin beta and darbepoetin alpha in anaemia associated with cancer, especially that attributable to cancer treatment.Health Technol Assess. 2007 Apr;11(13):1-202, iii-iv. doi: 10.3310/hta11130. Health Technol Assess. 2007. PMID: 17408534
-
What is the value of routinely testing full blood count, electrolytes and urea, and pulmonary function tests before elective surgery in patients with no apparent clinical indication and in subgroups of patients with common comorbidities: a systematic review of the clinical and cost-effective literature.Health Technol Assess. 2012 Dec;16(50):i-xvi, 1-159. doi: 10.3310/hta16500. Health Technol Assess. 2012. PMID: 23302507 Free PMC article.
References
-
- Wang H, Huang Z, Shen X, Lee Y, Song X, Shu C, et al. Rejuvenation of aged oocyte through exposure to young follicular microenvironment. Nat Aging. 2024;4(9):1194–210. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

