Mechanism of miR-107/HMOX1 axis in hepatic sinusoidal endothelial cells stimulated by ischemia-reperfusion injury
- PMID: 40671163
- PMCID: PMC12270030
- DOI: 10.1186/s41065-025-00495-4
Mechanism of miR-107/HMOX1 axis in hepatic sinusoidal endothelial cells stimulated by ischemia-reperfusion injury
Abstract
Background: Liver ischemia-reperfusion injury (IRI) is a common complication of diseases such as liver transplantation, hepatic resection, and hemorrhagic shock. This study aimed to elucidate the molecular mechanism of miR-107 affecting hepatic ischemia-reperfusion injury (IRI).
Methods: The expression changes of miR-107 during hepatic IRI were quantified using quantitative real-time PCR. Subsequently, in vitro cellular experiments were carried out to verify the role of miR-107 on hypoxia/reoxygenation (HR) through CCK-8, flow cytometer, and commercial kits. In terms of mechanism, it was determined that miR-107 had a regulatory relationship with target genes through luciferase reporter assay.
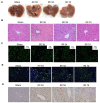
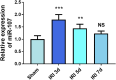
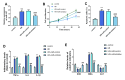
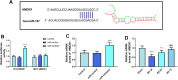
Results: In mouse liver IRI, miR-107 expression was increased while HMOX1 expression was decreased in liver tissues. In vitro cellular experiments, miR-107 inhibitors favored the alleviation of proliferation, apoptosis, inflammation, and oxidative stress in HR-damaged liver sinusoidal endothelial cells. In the molecular mechanism study, we determined that miR-107 could bind to HMOX1 and inhibit the HMOX1 expression. Low HMOX1 expression could eliminate the protective effect of miR-107 inhibitors.
Conclusion: MiR-107 expression was elevated during hepatic IRI and exacerbates hepatic injury by targeting HMOX1 inhibition.
Keywords: HMOX1; Hepatic IRI; Inflammation; Oxidation; miR-107.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval: This experiment was conducted with the approval of the Animal Ethics Committee of The Affiliated Hospital of Panzhihua University. All institutional and national guidelines for the care and use of laboratory animals were followed. Appropriate measures were taken to minimize the number and suffering of animals. Competing interests: The authors declare no competing interests.
Figures

Similar articles
-
LncRNA HMOX1 alleviates renal ischemia-reperfusion-induced ferroptotic injury via the miR-3587/HMOX1 axis.Cell Signal. 2024 Jul;119:111165. doi: 10.1016/j.cellsig.2024.111165. Epub 2024 Apr 6. Cell Signal. 2024. PMID: 38583746
-
miR-340-3p-modified bone marrow mesenchymal stem cell-derived exosomes inhibit ferroptosis through METTL3-mediated m6A modification of HMOX1 to promote recovery of injured rat uterus.Stem Cell Res Ther. 2024 Jul 29;15(1):224. doi: 10.1186/s13287-024-03846-6. Stem Cell Res Ther. 2024. PMID: 39075530 Free PMC article.
-
MEF2D Aggravates Hepatic Ischaemia-Reperfusion Injury by Transcriptionally Regulating CXCL1 Through Interacting With NAT10.Liver Int. 2025 Sep;45(9):e70315. doi: 10.1111/liv.70315. Liver Int. 2025. PMID: 40878991
-
Mesenchymal stem cells against intestinal ischemia-reperfusion injury: a systematic review and meta-analysis of preclinical studies.Stem Cell Res Ther. 2022 May 26;13(1):216. doi: 10.1186/s13287-022-02896-y. Stem Cell Res Ther. 2022. PMID: 35619154 Free PMC article.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
References
-
- Li J, Li J, Fang H, Yang H, Wu T, Shi X, et al. Isolongifolene alleviates liver ischemia/reperfusion injury by regulating AMPK-PGC1α signaling pathway-mediated inflammation, apoptosis, and oxidative stress. Int Immunopharmacol. 2022;113Pt A:109185. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

