Endothelial cell-secreted bone targeting exosomes promote angiogenesis coupling with osteogenesis via the PERK-ATF4-CRELD2 pathway
- PMID: 40671164
- PMCID: PMC12269303
- DOI: 10.1186/s13287-025-04449-5
Endothelial cell-secreted bone targeting exosomes promote angiogenesis coupling with osteogenesis via the PERK-ATF4-CRELD2 pathway
Abstract
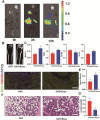
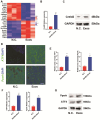
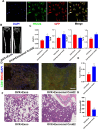
The role of endoplasmic reticulum (ER) stress in bone metabolism and the management of associated diseases has garnered significant interest. However, its role in regulating bone homeostasis and skeletal development remains largely unclear. Osteoblast development and bone formation are enhanced by a particular subtype of CD31hi endomucinhi (CD31hiEMCNhi) endothelium. However, it is still unclear how endothelial exosomes contribute to the production of CD31hiEMCNhi endothelium and bone formation. This research revealed that human umbilical vein endothelial cells (HUVECs)-exosomes (Exos) enhanced the formation of osteoblast and angiogenic effects in vitro. Furthermore, in mice treated with HUVECs-Exos, osteoblast production, and CD31hiEmcnhi vessels were significantly increased. The mechanism by which HUVECs-Exos CRELD2 improved angiogenesis coupling with osteogenesis involved triggering the PERK-ATF4-CRELD2 pathway's ER stress. As a result, HUVECs-Exos CRELD2 may be used as a potential bone metabolic disease nanodrug.
Keywords: Angiogenesis; CRELD2; Endoplasmic reticulum stress; Endothelium; Exosome; Osteogenesis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was approved by the Medical Research Ethics Board of the General Hospital of Southern Theater Command of PLA (title: exosomal CRELD2 promotes angiogenesis and osteogenesis via the PERK-ATF4-CRELD2 pathway. Approval number: No. 2023110101. Date of approval: 20231101). All methods were carried out in accordance with relevant guidelines and regulations. HUVECs were purchased from a commercial vendor (Procell, China), which information and ethics can be found on its official website (Information and ethics can be found on its official website ( https://www.procell.com.cn/view/2230.html ). Consent for publication: Not applicable. Competing interests: The authors declare that they have no conflicts of interest. The authors declare that they have not use AI-generated work in this manuscript.
Figures




Similar articles
-
Hypoxic mesenchymal stem cell-derived exosomes promote bone fracture healing by the transfer of miR-126.Acta Biomater. 2020 Feb;103:196-212. doi: 10.1016/j.actbio.2019.12.020. Epub 2019 Dec 17. Acta Biomater. 2020. PMID: 31857259
-
Divergent effects of premineralization and prevascularization on osteogenesis and vascular integration in humanized tissue engineered bone constructs.Acta Biomater. 2025 Jul 1;201:665-683. doi: 10.1016/j.actbio.2025.06.019. Epub 2025 Jun 11. Acta Biomater. 2025. PMID: 40514004
-
MiR-30d-5p Regulates Bone Remodeling and Vessel Remodeling in Osteoporosis by Targeting GRP78.FASEB J. 2025 Jul 15;39(13):e70781. doi: 10.1096/fj.202500345R. FASEB J. 2025. PMID: 40616398
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
References
-
- Ramasamy SK, Kusumbe AP, Itkin T, Gur-Cohen S, Lapidot T, Adams RH. Regulation of hematopoiesis and osteogenesis by blood vessel-derived signals. Annu Rev Cell Dev Biol. 2016;32:649–75. - PubMed
-
- Lei H, Guo W, Pan Y, Lu X, Zhang Q. LOX-1 regulation of H-type vascular endothelial cell regeneration in hyperglycemia. Acta Diabetol. 2024;61(4):515–24. - PubMed
MeSH terms
Substances
Grants and funding
- 2024A03J0690/Guangzhou Municipal Science and Technology Project
- 2023A03J0693/Guangzhou Science, Technology and Innovation Commission
- 2024A03J0634/Guangzhou Municipal Science and Technology Project
- 2022A1515010307/Natural Science Foundation of Guangdong Province
- 2024A1515012050/Natural Science Foundation of Guangdong Province
LinkOut - more resources
Full Text Sources
Research Materials

