Asymmetric Micro-Evolutionary Responses in a Warming World: Heat-Driven Adaptation Enhances Metal Tolerance in a Planktonic Rotifer, but Not Vice Versa
- PMID: 40671644
- PMCID: PMC12268377
- DOI: 10.1111/gcb.70347
Asymmetric Micro-Evolutionary Responses in a Warming World: Heat-Driven Adaptation Enhances Metal Tolerance in a Planktonic Rotifer, but Not Vice Versa
Abstract
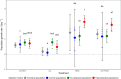
The resilience of natural populations in the face of global environmental change is determined by their ability to adapt to multiple, often interacting, stressors. Microevolutionary adaptation to one stressor can either enhance or reduce tolerance to other stressors. In the context of climate change, it is crucial to understand the effect of warming on the tolerance of organisms to additional environmental challenges. Conversely, adaptation to localized stressors, such as pollution, may also affect an organism's capacity to withstand climate change. Here, we investigate how prior adaptation to either high temperature or copper (Cu) contamination influences subsequent tolerance to the other stressor in populations of the freshwater zooplanktonic rotifer Brachionus calyciflorus (Pallas, 1766). Using an experimental evolution approach, we subjected populations to either gradually increasing Cu levels, elevated temperature, or control conditions over multiple generations. Subsequently, we conducted a common garden experiment to assess the effect of selection history on population performance. We found that heat-adapted populations exhibited increased tolerance to Cu, whereas Cu-adapted populations showed no enhanced tolerance to high temperatures. This form of "asymmetric cross-adaptation" is likely driven by selection for generalized stress responses associated with heat adaptation, while Cu adaptation selected for more specialized detoxification mechanisms with limited cross-protection. These findings suggest that the legacy of warming may enhance population tolerance to other stressors, whereas the benefits of adaptation to local pollution may be more constrained. Our study highlights the need to assess the generality of such patterns across taxa and stressor combinations, as this knowledge could inform environmental management strategies in multi-stressor contexts.
Keywords: climate change; copper toxicity; cross‐adaptation; cross‐tolerance; heat stress; micro‐evolutionary adaptation; pollution.
Global Change Biology© 2025 The Author(s). Global Change Biology published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Bates, D. , Mächler M., Bolker B., and Walker S.. 2015. “Fitting Linear Mixed‐Effects Models Using lme4.” Journal of Statistical Software 67: 1–48. 10.18637/jss.v067.i01. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

