Ginger-derived vesicle-like nanoparticles loaded with curcumin to alleviate ionizing radiation-induced intestinal damage via gut microbiota regulation
- PMID: 40671673
- PMCID: PMC12279272
- DOI: 10.1080/19490976.2025.2531210
Ginger-derived vesicle-like nanoparticles loaded with curcumin to alleviate ionizing radiation-induced intestinal damage via gut microbiota regulation
Abstract
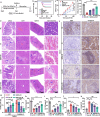
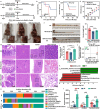
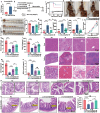
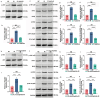
Emerging insights have been approached that gut microbiota act as a critical regulator for ionizing radiation (IR)-induced damage. Herein, an available strategy has been explored to shape gut microbiota for radioprotection by loading curcumin (Cur) into ginger-derived vesicle-like nanoparticles (GDNs). Engineered biomimetic nanovesicles (GDN-Cur) exhibited superb stability in the gastrointestinal tract, thereby significantly enhancing the oral bioavailability of Cur. Consequently, the intrinsic antioxidative, anti-inflammatory, and anti-apoptotic properties of GDNs and Cur granted this nanosystem exceptional protective effect against IR-induced injuries, especially in mitigating intestinal damage. Particularly, the dysbacteriosis triggered by IR could be counteracted through the oral administration of GDN-Cur, resulting in gut microbiota regulation-mediated syndrome mitigation. Furthermore, elevated abundances of Akkermansia muciniphila (A. muciniphila), a bacterial strain of Akkermansia taxa responsive to GDN-Cur, especially their supernatants, were associated with post-radiation protection of intestinal function. This beneficial effect was attributed to the identified radioprotective metabolites secreted by A. muciniphila, such as tanespimycin (17-AAG), which was demonstrated to deactivate AKT/NF-κB signaling pathway. These findings reveal the impact of plant products on radioprotective microbes and metabolites to target host processes and alleviate IR-induced intestinal damage, shedding light on new insights in the development of novel radioprotectants.
Keywords: Ionizing radiation-induced intestinal damage; curcumin; ginger-derived vesicle-like nanoparticles; gut microbiota; metabolites; radioprotection.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures





References
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
