2D-to-3D transformations of a covalent organic framework via post-synthetic crosslinking
- PMID: 40671758
- PMCID: PMC12261336
- DOI: 10.1039/d5sc02492g
2D-to-3D transformations of a covalent organic framework via post-synthetic crosslinking
Abstract
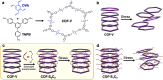
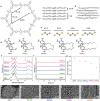
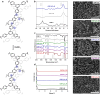
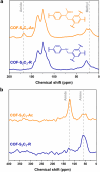
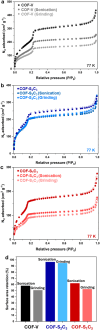
Two-dimensional (2D) covalent organic frameworks (COFs) are easier to synthesize and functionalize than their three-dimensional (3D) counterparts, but the 2D frameworks lack stability due to weak non-covalent interactions that maintain the layered structure. Herein, we provide a post-synthetic strategy to covalently crosslink the independent sheets of 2D COFs while preserving the crystallinity and porosity of the materials. The crosslinked frameworks show greatly enhanced mechanical stability compared to the parent 2D frameworks, retaining more than 90% of the original Brunauer-Emmett-Teller (BET) surface area when subjected to extensive sonication or grinding. Further, crosslinking enables the reduction of the imine linkages with sodium borohydride while preserving crystallinity and porosity, which has yet to be shown for 2D COFs. Finally, the imine linkages on a crosslinked framework were first reduced and then reacted with an acyl chloride, establishing a general approach to framework functionalization. This post-synthetic crosslinking approach stabilizes 2D frameworks and opens access to amine linkages in these materials, thus increasing hydrolytic stability and potential functionalization as selective adsorbents.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

References
-
- Lyle S. J. Waller P. J. Yaghi O. M. Trends Chem. 2019;1:172–184. doi: 10.1016/j.trechm.2019.03.001. - DOI
LinkOut - more resources
Full Text Sources

