Incretin-Based Therapies in Alzheimer's and Parkinson's Disease: Advancing Neuroprotection With Dual and Triple Agonists-A Review
- PMID: 40671844
- PMCID: PMC12264087
- DOI: 10.1002/hsr2.71065
Incretin-Based Therapies in Alzheimer's and Parkinson's Disease: Advancing Neuroprotection With Dual and Triple Agonists-A Review
Abstract
Background: Alzheimer's disease (AD) and Parkinson's disease (PD) are progressive neurodegenerative disorders with significant cognitive and motor impairments, affecting millions globally. Current treatments offer limited efficacy, prompting the exploration of new therapeutic approaches.
Aim: To discuss the intricate relationship between incretin and insulin signaling pathways and their relevance to the pathogenesis and treatment of Alzheimer's and Parkinson's diseases.
Methods: A comprehensive literature review was conducted using a variety of search engines, including Google Scholar, PubMed Central, Scopus, Web of Science, and others.
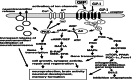
Results: Emerging evidence highlights disrupted insulin signaling in AD and, to a lesser extent, in PD, suggesting that insulin plays a key neuroprotective role. Incretins, such as GLP-1 and GIP, which enhance insulin signaling, have shown potential in preclinical and clinical studies. Incretin-based therapies, particularly GLP-1/GIP receptor agonists, have demonstrated promising effects by addressing several pathological processes, including oxidative stress, inflammation, misfolded protein aggregation, and insulin resistance. Dual agonists like DA-CH3, DA5-CH, and DA4-JC have proven superior in crossing the blood-brain barrier and offering improved neuroprotection in comparison with conventional GLP-1 agonists. Triple agonists provide even greater neuroprotective benefits, highlighting their potential as disease-modifying therapies for AD and PD.
Conclusion: While GLP-1 and GIP analogs hold promise in modulating early neurodegenerative processes, their efficacy likely depends on timely intervention before permanent neuronal damage occurs.
Keywords: Alzheimer disease; Parkinson disease; glucagon‐like peptide1 receptors, glucose‐dependent insolinotropic polypeptide; incretin signaling; insulin signaling; therapeutic targets.
© 2025 The Author(s). Health Science Reports published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Bourdenx M., Koulakiotis N. S., Sanoudou D., Bezard E., Dehay B., and Tsarbopoulos A., “Protein Aggregation and Neurodegeneration in Prototypical Neurodegenerative Diseases: Examples of Amyloidopathies, Tauopathies and Synucleinopathies,” Progress in Neurobiology 155 (2017): 171–193. - PubMed
-
- Gago M., Machado A., and Rocha S., “Current Clinical Approaches in Neurodegenerative Diseases.” Handbook of Innovations in Central Nervous System Regenerative Medicine (Elsevier, 2020), 79–124.
LinkOut - more resources
Full Text Sources

