An azo substituted quinoline-malononitrile enzyme-activable aggregation-induced emission nanoprobe for hypoxia imaging
- PMID: 40671929
- PMCID: PMC12262007
- DOI: 10.1002/smo.20240028
An azo substituted quinoline-malononitrile enzyme-activable aggregation-induced emission nanoprobe for hypoxia imaging
Abstract
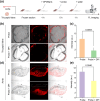
The development of efficient aggregation-induced emission (AIE) active probes is crucial for disease diagnosis, particularly for tumors and cardiovascular diseases. Current AIE-active probes primarily focus on improving their water solubility to resist aggregation, thereby achieving an initial fluorescence-off state. However, the complex biological environment can cause undesirable aggregation, resulting in false signals. To address this issue, we have ingeniously introduced an azo group into the AIE luminogen (AIEgen), developing a reductase-activated AIE probe, Azo-quinoline-malononitrile (QM)-PN, for imaging hypoxic environments. In this probe, the azo group promotes intramolecular motion through rapid E/Z isomerization, causing the excited state energy to dissipate via non-radiative decay, thus turning off the initial fluorescence. In the presence of reductase, Azo-QM-PN is reduced and cleaved to produce the hydrophobic AIEgen NH2-QM-PN, which subsequently aggregates and generates an in situ AIE signal, thereby imaging the hypoxic environment with reductase. Encapsulation of Azo-QM-PN with DSPE-PEG2000 results in the formation of the nanoprobe Azo-QM-PN NPs, which can effectively penetrate cell membranes, specifically illuminate tumor cells, monitor fluctuations in azo reductase levels, and deeply penetrate and image multicellular tumor spheroids, demonstrating potential for hypoxic tumor imaging. Additionally, the nanoprobe Azo-QM-PN NPs can selectively image hypoxic atherosclerotic plaque tissues, showing potential for detecting atherosclerosis. Therefore, in this study, we successfully developed an enzyme-activated AIE probe for imaging hypoxic environments, laying the foundation for further clinical applications.
Keywords: AIE‐active; fluorescent probe; hypoxia imaging.
© 2024 The Author(s). Smart Molecules published by John Wiley & Sons Australia, Ltd on behalf of Dalian University of Technology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Zhu Z., Chen X., Liao H., Li L., Yang H., Wang Q., Zhu W. H., Aggregate 2024, 5, e526.
-
- Tu Y., Zhao Z., Lam J. W. Y., Tang B. Z., Matter 2021, 4, 338.
-
- Hu Z., Fang C., Li B., Zhang Z., Cao C., Cai M., Su S., Sun X., Shi X., Li C., Zhou T., Zhang Y., Chi C., He P., Xia X., Chen Y., Gambhir S. S., Cheng Z., Tian J., Nat. Biomed. Eng. 2020, 4, 259. - PubMed
LinkOut - more resources
Full Text Sources
