This is a preprint.
Emergent microtubule properties in a model of filament turnover and nucleation
- PMID: 40671949
- PMCID: PMC12265573
Emergent microtubule properties in a model of filament turnover and nucleation
Update in
-
Emergent microtubule properties in a model of filament turnover and nucleation.J Theor Biol. 2025 Aug 30;616:112254. doi: 10.1016/j.jtbi.2025.112254. Online ahead of print. J Theor Biol. 2025. PMID: 40889592
Abstract
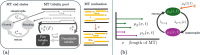
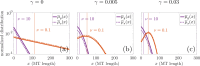
Microtubules (MTs) are dynamic protein filaments essential for intracellular organization and transport, particularly in long-lived cells such as neurons. The plus and minus ends of neuronal MTs switch between growth and shrinking phases, and the nucleation of new filaments is believed to be regulated in both healthy and injury conditions. We propose stochastic and deterministic mathematical models to investigate the impact of filament nucleation and length-regulation mechanisms on emergent properties such as MT lengths and numbers in living cells. We expand our stochastic continuous-time Markov chain model of filament dynamics to incorporate MT nucleation and capture realistic stochastic fluctuations in MT numbers and tubulin availability. We also propose a simplified partial differential equation (PDE) model, which allows for tractable analytical investigation into steady-state MT distributions under different nucleation and length-regulating mechanisms. We find that the stochastic and PDE modeling approaches show good agreement in predicted MT length distributions, and that both MT nucleation and the catastrophe rate of large-length MTs regulate MT length distributions. In both frameworks, multiple mechanistic combinations achieve the same average MT length. The models proposed can predict parameter regimes where the system is scarce in tubulin, the building block of MTs, and suggest that low filament nucleation regimes are characterized by high variation in MT lengths, while high nucleation regimes drive high variation in MT numbers. These mathematical frameworks have the potential to improve our understanding of MT regulation in both healthy and injured neurons.
Keywords: microtubule turnover; nucleation; stochastic modeling.
Figures



Similar articles
-
Emergent microtubule properties in a model of filament turnover and nucleation.J Theor Biol. 2025 Aug 30;616:112254. doi: 10.1016/j.jtbi.2025.112254. Online ahead of print. J Theor Biol. 2025. PMID: 40889592
-
Nucleation feedback can drive establishment and maintenance of biased microtubule polarity in neurites.ArXiv [Preprint]. 2025 Aug 28:arXiv:2506.12209v2. ArXiv. 2025. PMID: 40909153 Free PMC article. Preprint.
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
References
-
- Mitchison Tim and Kirschner Marc. Dynamic instability of microtubule growth. Nature, 312(5991):237–242, 1984. - PubMed
-
- Teixidó-Travesa Neus, Roig Joan, and Lüders Jens. The where, when and how of microtubule nucleation–one ring to rule them all. Journal of Cell Science, 125(19):4445–4456, 2012. - PubMed
-
- Desai Arshad and Mitchison Timothy J. Microtubule polymerization dynamics. Annual Review of Cell and Developmental Biology, 13(1):83–117, 1997. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
