Complete Remission With Fourth-Line Immunotherapy After Chemotherapy Failure in a Lung Cancer Patient With Severe Autoimmune Disease: An Unexpected Turn in Oncologic Management
- PMID: 40672009
- PMCID: PMC12265009
- DOI: 10.7759/cureus.86116
Complete Remission With Fourth-Line Immunotherapy After Chemotherapy Failure in a Lung Cancer Patient With Severe Autoimmune Disease: An Unexpected Turn in Oncologic Management
Abstract
The indication for nivolumab in patients with advanced non-small cell lung cancer (NSCLC) who have progressed to first-line platinum-based systemic therapy was one of the first indications for immunotherapy approved by regulatory agencies. However, it is generally the case that patients with autoimmune diseases (AIDs) are excluded from studies due to the risk of immune exacerbations and a higher rate of immune-related adverse effects. This deprives these patients of the potential benefits they could obtain from immunotherapy, especially in those cases with favorable biomarkers of a good response. In this study, we present a clinical case of a patient with rapidly progressive multiple sclerosis (MS) of years of evolution, who obtained an impressive response to immunotherapy as a last therapeutic option, remaining cancer-free to date.
Keywords: autoimmune disease; complete response; immune related adverse events; immunotherapy; lung cancer.
Copyright © 2025, Herrero Rivera et al.
Conflict of interest statement
Human subjects: Informed consent for treatment and open access publication was obtained or waived by all participants in this study. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: Daniel Herrero Rivera declare(s) Support for attending meetings and/or travel from Bristol Myers Squibb, Ipsen, Lilly, Roche. Daniel Herrero Rivera declare(s) personal fees from AstraZeneca, Pfizer and Roche. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures
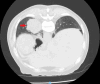

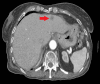

Similar articles
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Nivolumab for adults with Hodgkin's lymphoma (a rapid review using the software RobotReviewer).Cochrane Database Syst Rev. 2018 Jul 12;7(7):CD012556. doi: 10.1002/14651858.CD012556.pub2. Cochrane Database Syst Rev. 2018. PMID: 30001476 Free PMC article.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of topotecan for ovarian cancer.Health Technol Assess. 2001;5(28):1-110. doi: 10.3310/hta5280. Health Technol Assess. 2001. PMID: 11701100
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
-
[Volume and health outcomes: evidence from systematic reviews and from evaluation of Italian hospital data].Epidemiol Prev. 2013 Mar-Jun;37(2-3 Suppl 2):1-100. Epidemiol Prev. 2013. PMID: 23851286 Italian.
References
-
- Safety and efficacy of immune checkpoint inhibitors in cancer patients and preexisting autoimmune disease: a systematic review and meta-analysis in non-small-cell lung cancer. Aung WY, Lee CS, Morales J, Rahman H, Seetharamu N. http://www.sciencedirect.com/science/article/pii/S1525730423001080. Clin Lung Cancer. 2023;24:598–612. - PubMed
-
- Immune checkpoint inhibitors therapies in patients with cancer and preexisting autoimmune diseases: A meta-analysis of observational studies. Xie W, Huang H, Xiao S, Fan Y, Deng X, Zhang Z. Autoimmun Rev. 2020;19:102687. - PubMed
-
- Risk and survival of patients with non-small cell lung cancer and pre-existing autoimmune disorders receiving immune checkpoint blockade therapy: Survival analysis with inverse probability weighting from a nationwide, multi-institutional, retrospective study (NEJ047) Asao T, Shukuya T, Uemura K, et al. Lung Cancer. 2024;194:107894. - PubMed
-
- Are patients with autoimmune disorders eligible for immunotherapy? Madama D, Pego A. Pulmonology. 2021;27:264–266. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
