Efficacy of Non-opioid Analgesics in the Management of Postoperative Pain After Major Abdominal Surgery: A Comprehensive Review and Meta-Analysis of Randomized Controlled Trials
- PMID: 40672017
- PMCID: PMC12266804
- DOI: 10.7759/cureus.86157
Efficacy of Non-opioid Analgesics in the Management of Postoperative Pain After Major Abdominal Surgery: A Comprehensive Review and Meta-Analysis of Randomized Controlled Trials
Abstract
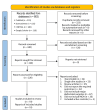
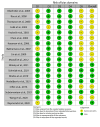
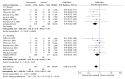
Postoperative pain (POP) is one of the leading clinical challenges of patients undergoing major surgeries, including abdominal surgeries. Opioid analgesics are considered the gold standard for POP. However, their use is associated with a high incidence of adverse events, including nausea and vomiting, which has prompted clinicians to look for alternative regimens that are not opioid-based. This review aims to provide an overview of the existing evidence regarding the effectiveness of non-opioid analgesics (NOAs) in the management of POP in patients who have undergone major abdominal surgeries. A comprehensive search was conducted on four databases: Google Scholar, PubMed, CENTRAL, and Science Direct. The studies that met the inclusion criteria were then included in the review. The reported outcomes were pooled using the Review Manager software (RevMan 5.4, The Cochrane Collaboration, London, UK). The literature search identified 657 articles, among which 18 were included in the review according to the inclusion criteria. Our study found that the mean postoperative opioid consumption was significantly lower among individuals treated with non-opioid analgesics than with opioid analgesics SMD -1.88; 95% CI (-2.40, -1.36); p < 0.0001). Further analysis showed that the mean opioid consumption was also lower in those who received NSAIDs and other atypical analgesics (SMD -2.24; 95% CI (-2.94, -1.55); p < 0.00001) and (SMD -1.18; 95% CI (-2.18, -0.17); p = 0.02), respectively. However, in those who received paracetamol, the mean opioid consumption was comparable to that of controls (SMD -1.09; 95% CI (-2.21, 0.03); p = 0.06). Secondly, our study found that the incidence of opioid-related nausea was reduced in patients who received NOA than in controls (OR 0.38; 95% CI (0.22, 0.66); p = 0.0005). However, the incidence of vomiting was equivalent across both groups (OR 0.64; 95% CI (0.39, 1.04); p = 0.07). This study found that NOAs are good adjuvants in pain management in patients undergoing major abdominal surgery. They aid in reducing the dosage of opioids required for adequate analgesia and thus also reduce the incidence of some of the related adverse events.
Keywords: (nsaid) non-steroidal anti-inflammatory drugs; analgesia; non-opioid analgesics; postoperative pain; surgery; systematic review and meta analysis.
Copyright © 2025, Ponappan et al.
Conflict of interest statement
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures


Similar articles
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
-
Oral non-steroidal anti-inflammatory drugs versus other oral analgesic agents for acute soft tissue injury.Cochrane Database Syst Rev. 2015 Jul 1;(7):CD007789. doi: 10.1002/14651858.CD007789.pub2. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2020 Aug 12;8:CD007789. doi: 10.1002/14651858.CD007789.pub3. PMID: 26130144 Updated.
-
Oxycodone for cancer-related pain.Cochrane Database Syst Rev. 2022 Jun 9;6(6):CD003870. doi: 10.1002/14651858.CD003870.pub7. Cochrane Database Syst Rev. 2022. PMID: 35679121 Free PMC article.
-
Ketorolac for postoperative pain in children.Cochrane Database Syst Rev. 2018 Jul 7;7(7):CD012294. doi: 10.1002/14651858.CD012294.pub2. Cochrane Database Syst Rev. 2018. PMID: 29981164 Free PMC article.
-
Nonsteroidal anti-inflammatory drugs (NSAIDs) and non-opioids for acute renal colic.Cochrane Database Syst Rev. 2015 Jun 29;2015(6):CD006027. doi: 10.1002/14651858.CD006027.pub2. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2025 Mar 14;3:CD006027. doi: 10.1002/14651858.CD006027.pub3. PMID: 26120804 Free PMC article. Updated.
References
-
- An estimation of the global volume of surgery: a modelling strategy based on available data. Weiser TG, Regenbogen SE, Thompson KD, Haynes AB, Lipsitz SR, Berry WR, Gawande AA. Lancet. 2008;372:139–144. - PubMed
-
- Pain intensity on the first day after surgery: a prospective cohort study comparing 179 surgical procedures. Gerbershagen HJ, Aduckathil S, van Wijck AJ, Peelen LM, Kalkman CJ, Meissner W. Anesthesiology. 2013;118:934–944. - PubMed
-
- Patient controlled opioid analgesia versus conventional opioid analgesia for postoperative pain. Hudcova J, McNicol E, Quah C, Lau J, Carr DB. Cochrane Database Syst Rev. 2006:0. - PubMed
-
- Patient-controlled analgesia in the management of postoperative pain. Momeni M, Crucitti M, De Kock M. Drugs. 2006;66:2321–2337. - PubMed
-
- Non-opioid analgesics in adults after major surgery: systematic review with network meta-analysis of randomized trials. Martinez V, Beloeil H, Marret E, Fletcher D, Ravaud P, Trinquart L. Br J Anaesth. 2017;118:22–31. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
