Metformin Activation of Sirtuin 3 Signaling Regulates Mitochondrial Function Improves Diabetes-Associated Cognitive Impairment
- PMID: 40672059
- PMCID: PMC12266075
- DOI: 10.2147/DMSO.S516173
Metformin Activation of Sirtuin 3 Signaling Regulates Mitochondrial Function Improves Diabetes-Associated Cognitive Impairment
Abstract
Context: Diabetes-associated cognitive impairment (DACD) is a prevalent complication of diabetes mellitus, with a strong correlation to both the severity and duration of the disease. While metformin has demonstrated a significant impact on mitigating DACD, the precise mechanisms underlying its therapeutic effects remain inadequately understood.
Objective: This study aims to examine the protective effects of metformin (MET) on DACD and to elucidate the underlying mechanisms involved.
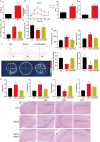
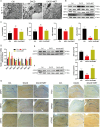
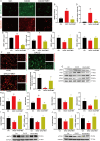
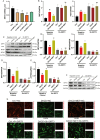
Materials and methods: C57BL/6J male mice from in vivo animal experiments established DACD by high-fat diet (HFD) for 12 weeks, combined with intraperitoneal injection of low-dose streptozotocin (STZ, 40 mg/kg). Subsequently, DACD mice were administered MET for 2 months. The expression levels of proteins related to mitochondrial function were analyzed using immunohistochemical staining, immunofluorescence double staining, qRT-PCR, and Western blot. Furthermore, the mechanism underlying the improvement of DACD by MET was validated by using the Sirtuin 3 (SIRT3) agonist resveratrol (RES), the inhibitor 3-TYP, and sh-SIRT3 on astrocytes.
Results: Our findings indicate that MET significantly ameliorated mitochondrial dysfunction in DACD mice, accompanied by an upregulation of SIRT3 expression. Furthermore, comparable results were noted with the SIRT3 agonist RES. Meanwhile, suppressing SIRT3 expression via sh-SIRT3 or SIRT3 inhibitor 3-TYP in astrocytes largely abolished MET's ability to restore mitochondrial function.
Conclusion: It has been demonstrated that MET ameliorates mitochondrial dysfunction by activating the SIRT3 signaling pathway to rescue DACD.
Keywords: astrocytes; diabetes-associated cognitive dysfunction; metformin; mitochondria; sirtuin 3.
© 2025 An et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources
Miscellaneous

