CDK12 inhibition enhances oxaliplatin efficacy in gastric cancer by suppressing the MAPK signaling pathway
- PMID: 40672076
- PMCID: PMC12261016
- DOI: 10.21037/jgo-2025-392
CDK12 inhibition enhances oxaliplatin efficacy in gastric cancer by suppressing the MAPK signaling pathway
Abstract
Background: Gastric cancer (GC) is a leading cause of cancer-related mortality worldwide. Oxaliplatin (OXA) based therapy plus/minus targeted therapy and immune check point inhibitors is the standard first-line treatment for advanced GC; however, its clinical efficacy is often hindered by the development of drug resistance. Cyclin-dependent kinase 12 (CDK12), a transcriptional regulator linked to DNA repair, plays a crucial role in transcription, cancer progression as well as drug resistance, where its exact role is unclear. In this study, we will investigate the role of CDK12 in GC progression and its potential as a therapeutic target specifically to enhance the efficacy of OXA.
Methods: CDK12 expression in GC tissues was analyzed by quantitative polymerase chain reaction (qPCR) and tissue microarrays (TMAs). A Kaplan-Meier survival analysis was conducted to assess the relationship between CDK12 levels and clinical outcomes. The effect of the CDK12 inhibitor combined with OXA was evaluated through in vivo and in vitro models. RNA-sequencing and western blots were used to investigate the molecular mechanisms of CDK12 inhibitor sensitizing OXA.
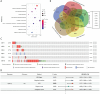
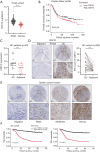
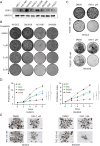
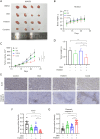
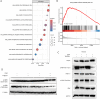
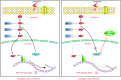
Results: CDK12 exhibited significant amplification frequency in GC. The Mendelian-randomization analysis revealed a positive causal association between elevated CDK12 expression and an increased risk of GC. Additionally, CDK12 was significantly overexpressed in GC tissues compared with adjacent normal tissues, and its high expression was significantly associated with a worse prognosis. The functional assays revealed that combining the CDK12 inhibitor THZ531 with OXA synergistically suppressed GC cell proliferation, induced apoptosis, and reduced colony formation in vitro, while substantially inhibiting tumor growth in xenograft models. Mechanistically, CDK12 inhibition disrupted MAPK signaling, leading to enhanced OXA-induced DNA damage and potentiated anti-tumor effects.
Conclusions: Our findings suggest that CDK12 inhibition may represent a promising strategy for overcoming OXA resistance and improving GC treatment outcomes.
Keywords: Gastric cancer (GC); cell cycle; combination therapy; cyclin-dependent kinase 12 (CDK12); oxaliplatin (OXA).
Copyright © 2025 AME Publishing Company. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-2025-392/coif). The authors have no conflicts of interest to declare.
Figures
References
-
- Zheng RS, Chen R, Han BF, et al. Cancer incidence and mortality in China, 2022. Zhonghua Zhong Liu Za Zhi 2024;46:221-31. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
