This is a preprint.
Time-resolved functional connectivity during visuomotor graph learning
- PMID: 40672225
- PMCID: PMC12265615
- DOI: 10.1101/2024.07.04.602005
Time-resolved functional connectivity during visuomotor graph learning
Abstract
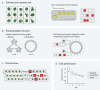
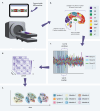
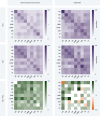
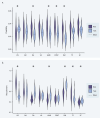
Humans naturally attend to patterns that emerge in our perceptual environments, building mental models that allow future experiences to be processed more effectively and efficiently. Perceptual events and statistical relations can be represented as nodes and edges in a graph. Recent work in graph learning has shown that human behavior is sensitive to graph topology, but little is known about how that topology might elicit distinct neural responses during learning. Here, we address this knowledge gap by applying time-resolved network analyses to fMRI data collected during a visuomotor graph learning task. We assess neural signatures of learning on two types of structures: modular and non-modular lattice graphs. We find that task performance is supported by a highly flexible visual system, relatively stable brain-wide community structure, cohesiveness within the dorsal attention, limbic, default mode, and subcortical systems, and an increasing degree of integration between the visual and ventral attention systems. Additionally, we find that the time-resolved connectivity of the limbic, default mode, temporoparietal, and subcortical systems is associated with enhanced performance on modular graphs but not on lattice-like graphs. These findings provide evidence for the differential processing of statistical patterns with distinct underlying graph topologies. Our work highlights the similarities between the neural correlates of graph learning and those of statistical learning.
Figures


Similar articles
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
"In a State of Flow": A Qualitative Examination of Autistic Adults' Phenomenological Experiences of Task Immersion.Autism Adulthood. 2024 Sep 16;6(3):362-373. doi: 10.1089/aut.2023.0032. eCollection 2024 Sep. Autism Adulthood. 2024. PMID: 39371355
-
The Lived Experience of Autistic Adults in Employment: A Systematic Search and Synthesis.Autism Adulthood. 2024 Dec 2;6(4):495-509. doi: 10.1089/aut.2022.0114. eCollection 2024 Dec. Autism Adulthood. 2024. PMID: 40018061 Review.
-
Sexual Harassment and Prevention Training.2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 36508513 Free Books & Documents.
-
Fabricating mice and dementia: opening up relations in multi-species research.In: Jenkins N, Jack-Waugh A, Ritchie L, editors. Multi-Species Dementia Studies. Bristol (UK): Bristol University Press; 2025 Feb 25. Chapter 2. In: Jenkins N, Jack-Waugh A, Ritchie L, editors. Multi-Species Dementia Studies. Bristol (UK): Bristol University Press; 2025 Feb 25. Chapter 2. PMID: 40690569 Free Books & Documents. Review.
References
-
- Vitevitch Michael S. and Luce Paul A.. “Probabilistic Phonotactics and Neighborhood Activation in Spoken Word Recognition”. In: Journal of Memory and Language 40.3 (Apr. 1999), pp. 374–408. ISSN: 0749596X. DOI: 10.1006/jmla.1998.2618. URL: https://linkinghub.elsevier.com/retrieve/pii/S0749596X98926183. - DOI
-
- Schapiro Anna C. and Turk-Browne Nicholas B.. “Statistical Learning”. In: Brain Mapping. Vol. 3. Elsevier, 2015, pp. 501–506. ISBN: 9780123970251. DOI: 10.1016/B978-0-12-397025-1.00276-1. URL: https://linkinghub.elsevier.com/retrieve/pii/B9780123970251002761. - DOI
-
- Fiser József and Aslin Richard N.. “Statistical learning of higher-order temporal structure from visual shape sequences.” In: Journal of Experimental Psychology: Learning, Memory, and Cognition 28.3 (2002), pp. 458–467. ISSN: 1939–1285. DOI: 10.1037/0278-7393.28.3.458. URL: http://doi.apa.org/getdoi.cfm?doi=10.1037/0278-7393.28.3.458. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
