This is a preprint.
Single-nucleotide Resolution Epitranscriptomic Profiling Uncovers Dynamic m6A Regulation in Bovine Preimplantation Development
- PMID: 40672241
- PMCID: PMC12265534
- DOI: 10.1101/2025.07.07.663558
Single-nucleotide Resolution Epitranscriptomic Profiling Uncovers Dynamic m6A Regulation in Bovine Preimplantation Development
Abstract
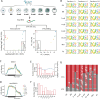
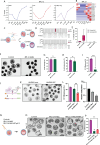
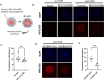
RNA N 6-methyladenosine (m6A) plays a crucial role in regulating gene expression during early embryonic development. However, the m6A dynamics at single-nucleotide resolution in preimplantation development remain uncharacterized, and the functional significance of site specific m6A modifications in key developmental regulators is largely unknown. Here, using SAC-seq, a single-base resolution, antibody-independent m6A profiling method, we generate the first comprehensive m6A landscape in bovine oocytes and preimplantation embryos. We identify a previously uncharacterized m6A site in RPL12 transcript that is essential for embryonic development. Loss of m6A at this site leads to reduced protein synthesis, disrupted expression of translation-related genes, and impaired zygotic genome activation and blastocyst formation. Notably, supplementation with wild-type RPL12 mRNA fails to rescue the developmental arrest, indicating that m6A regulation extends beyond transcript abundance. Our findings provide a valuable resource of m6A at single-nucleotide resolution in mammalian embryogenesis and uncover a critical mechanism by which precise, site-specific m6A regulates translation and developmental competence in early embryos.
Conflict of interest statement
Competing interests The authors declare no competing interests. P.K.J. is a co-founder of CasNx, LLC and CRISPR, LLC.
Figures



Similar articles
-
The RNA m6A landscape during human oocyte-to-embryo transition.EMBO J. 2025 Jul;44(14):4150-4180. doi: 10.1038/s44318-025-00474-5. Epub 2025 Jun 4. EMBO J. 2025. PMID: 40467862 Free PMC article.
-
Clinical outcomes following selection of human preimplantation embryos with time-lapse monitoring: a systematic review.Hum Reprod Update. 2014 Sep-Oct;20(5):617-31. doi: 10.1093/humupd/dmu023. Epub 2014 Jun 2. Hum Reprod Update. 2014. PMID: 24890606
-
c-Abl Plays an Important Role in Mouse Preimplantation Embryo Development and the Dysregulation Associated With Decreased mTERT Expression.Mol Reprod Dev. 2025 Jun;92(6):e70039. doi: 10.1002/mrd.70039. Mol Reprod Dev. 2025. PMID: 40528788 Free PMC article.
-
Profiling and functional characterization of maternal mRNA translation during mouse maternal-to-zygotic transition.Sci Adv. 2022 Feb 4;8(5):eabj3967. doi: 10.1126/sciadv.abj3967. Epub 2022 Feb 2. Sci Adv. 2022. PMID: 35108058 Free PMC article.
-
Day 5 versus day 3 embryo biopsy for preimplantation genetic testing for monogenic/single gene defects.Cochrane Database Syst Rev. 2022 Nov 24;11(11):CD013233. doi: 10.1002/14651858.CD013233.pub2. Cochrane Database Syst Rev. 2022. PMID: 36423200 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
