This is a preprint.
A Bioelectrochemical Crossbar Architecture Screening Platform (BiCASP) for Extracellular Electron Transfer
- PMID: 40672258
- PMCID: PMC12265703
- DOI: 10.1101/2025.07.09.663982
A Bioelectrochemical Crossbar Architecture Screening Platform (BiCASP) for Extracellular Electron Transfer
Abstract
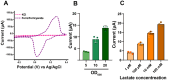
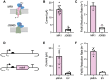
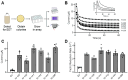
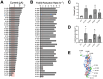
Electroactive microbes can be used as components in electrical devices to leverage their unique behavior for biotechnology, but they remain challenging to engineer because the bioelectrochemical systems (BES) used for characterization are low-throughput. To overcome this challenge, we describe the development of the Bioelectrochemical Crossbar Architecture Screening Platform (BiCASP), which allows for samples to be arrayed and characterized in individually addressable microwells. This device reliably reports on the current generated by electroactive bacteria on the minute time scale, decreasing the time for data acquisition by several orders of magnitude compared to conventional BES. Also, this device increased the throughput of screening engineered biological components in cells, quickly identifying mutants of the membrane protein wire MtrA in Shewanella oneidensis that retain the ability to support extracellular electron transfer (EET). BiCASP is expected to enable the design of new components for bioelectronics by supporting directed evolution of electroactive proteins.
Keywords: Arrayed microwells; Shewanella; bioelectrochemical systems; bioelectronics; crossbar architecture; decaheme protein; electrical multiplex; electroactive microbe; extracellular electron transfer; high-throughput screening.
Conflict of interest statement
Declaration of interests The authors declare no conflict of interest.
Figures

Similar articles
-
Identification of factors limiting the efficiency of transplanting extracellular electron transfer chains in Escherichia coli.Appl Environ Microbiol. 2025 Jun 18;91(6):e0068525. doi: 10.1128/aem.00685-25. Epub 2025 May 13. Appl Environ Microbiol. 2025. PMID: 40358241 Free PMC article.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Sexual Harassment and Prevention Training.2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 36508513 Free Books & Documents.
-
Behavioral interventions to reduce risk for sexual transmission of HIV among men who have sex with men.Cochrane Database Syst Rev. 2008 Jul 16;(3):CD001230. doi: 10.1002/14651858.CD001230.pub2. Cochrane Database Syst Rev. 2008. PMID: 18646068
References
-
- Cao Z., Xie Y., Lin J.L., Zhong S., Yan C., Yang Z., Li M., Zhou Z., Peng W., Qiu S., et al. (2024). Flexible Crossbar Molecular Devices with Patterned EGaIn Top Electrodes for Integrated All-Molecule-Circuit Implementation. Advanced Materials 36, 2406456. 10.1002/ADMA.202406456;PAGE:STRING:ARTICLE/CHAPTER. - DOI - PubMed
-
- Lightbody G., Haberland V., Browne F., Taggart L., Zheng H., Parkes E., and Blayney J.K. (2019). Review of applications of high-throughput sequencing in personalized medicine: Barriers and facilitators of future progress in research and clinical application. Brief Bioinform 20, 1795–1811. 10.1093/BIB/BBY051,. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
