This is a preprint.
Active Dissociation of Intracortical Spiking and High Gamma Activity
- PMID: 40672280
- PMCID: PMC12265708
- DOI: 10.1101/2025.07.10.663559
Active Dissociation of Intracortical Spiking and High Gamma Activity
Abstract
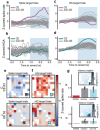
Cortical high gamma activity (HGA) is used in many scientific investigations, yet its biophysical source is a matter of debate. Two leading hypotheses are that HGA predominantly represents summed postsynaptic potentials or-more commonly- predominantly represents summed local spikes. If the latter were true, the nearest neurons to an electrode should contribute most to HGA recorded on that electrode. We trained subjects to decouple spiking from HGA on a single electrode using a brain-machine interface. Their ability to decouple them indicated that HGA is not primarily generated by summed local spiking. Instead, HGA correlated with neuronal population co-firing of neurons that were widely distributed across millimeters. The neuronal spikes that contributed more to this co-firing also contributed more to, and preceded, spike-triggered HGA. These results suggest that HGA arises predominantly from summed postsynaptic potentials triggered by synchronous co-firing of widely distributed neurons.
Figures






References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
