This is a preprint.
Nuclear and cytosolic J-domain proteins provide synergistic control of Hsf1 at distinct phases of the heat shock response
- PMID: 40672285
- PMCID: PMC12265559
- DOI: 10.1101/2025.04.14.648540
Nuclear and cytosolic J-domain proteins provide synergistic control of Hsf1 at distinct phases of the heat shock response
Update in
-
Nuclear and cytosolic J-domain proteins provide synergistic control of Hsf1 at distinct phases of the heat shock response.Elife. 2025 Sep 30;14:RP107157. doi: 10.7554/eLife.107157. Elife. 2025. PMID: 41025326 Free PMC article.
Abstract
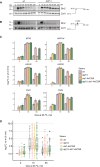
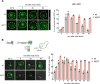
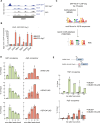
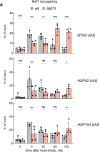
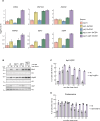
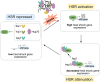
The heat shock response (HSR) is the major defense mechanism against proteotoxic stress in the cytosol and nucleus of eukaryotic cells. Initiation and attenuation of the response are mediated by stress-dependent regulation of heat shock transcription factors (HSFs). Saccharomyces cerevisiae encodes a single HSF (Hsf1), facilitating the analysis of HSR regulation. Hsf1 is repressed by Hsp70 chaperones under non-stress conditions, and becomes activated under proteotoxic stress, directly linking protein damage and its repair to the HSR. J-domain proteins (JDPs) are essential for targeting of Hsp70s to their substrates, yet the specific JDP(s) regulating Hsf1 and connecting protein damage to HSR activation remain unclear. Here we show that the yeast nuclear JDP Apj1 primarily controls the attenuation phase of the HSR by promoting Hsf1's displacement from heat shock elements in target DNA. In apj1Δ cells, HSR attenuation is significantly impaired. Additionally, yeast cells lacking both Apj1 and the major JDP Ydj1 exhibit increased HSR activation even in non-stress conditions, indicating their distinct regulatory roles. Apj1's role in both nuclear protein quality control and Hsf1 regulation underscores its role in directly linking nuclear proteostasis to HSR regulation. Together these findings establish the nucleus as key stress-sensing signaling hub.
Figures

References
-
- Aparicio O, Geisberg JV, Sekinger E, Yang A, Moqtaderi Z, Struhl K (2005) Chromatin immunoprecipitation for determining the association of proteins with specific genomic sequences in vivo. Curr Protoc Mol Biol Chapter 21: Unit 21 23 - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
