This is a preprint.
Unravelling the role of epigenetic regulators during embryonic development of Rhipicephalus microplus
- PMID: 40672298
- PMCID: PMC12265629
- DOI: 10.1101/2025.07.11.662657
Unravelling the role of epigenetic regulators during embryonic development of Rhipicephalus microplus
Abstract
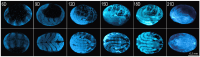
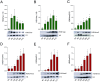
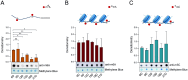
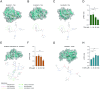
Epigenetic modifications are long-lasting changes to the genome that influence a cell's transcriptional potential, thereby altering its function. These modifications can trigger adaptive responses that impact protein expression and various cellular processes, including differentiation and growth. The primary epigenetic mechanisms identified to date include DNA and RNA methylation, histone modifications, and microRNA-mediated regulation of gene expression. The intricate crosstalk among these mechanisms makes epigenetics a compelling field for the development of novel control strategies, particularly through the use of epigenetic drugs targeting arthropod vectors such as ticks. In this study, we identified the Rhipicephalus microplus orthologs of canonical histone-modifying enzymes, along with components of the machinery responsible for m5C and 6mA-DNA, and m6A-RNA methylations. We further characterized their transcriptional profiles and enzymatic activities during embryonic development. To explore the functional consequences of epigenetic regulation in R. microplus, we evaluated the effects of various epigenetic inhibitors on the BME26 tick embryonic cell line. Molecular docking simulations were performed to predict the binding mode of these inhibitors to tick enzymes, followed by in vitro assessment of their effects on cell viability and morphology. Tick cells exposed to these inhibitors exhibited phenotypic and molecular alterations. Notably, we observed higher levels of DNA methylation in the mitochondrial genome compared to nuclear DNA. Inhibition of DNA methylation using 5'-azacytidine (5'-AZA) was associated with increased activity of the mitochondrial electron transport chain and ATP synthesis, but reduced cellular proliferation. Our findings highlight the importance of epigenetic regulation during tick embryogenesis and suggest that targeting these pathways may offer a novel and promising strategy for tick control.
Figures


Similar articles
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Mapping DNA Methylation to Cardiac Pathologies Induced by Beta-Adrenergic Stimulation in a Large Panel of Mice.bioRxiv [Preprint]. 2024 Oct 26:2024.10.25.619688. doi: 10.1101/2024.10.25.619688. bioRxiv. 2024. Update in: Epigenetics. 2025 Dec;20(1):2524411. doi: 10.1080/15592294.2025.2524411. PMID: 39484431 Free PMC article. Updated. Preprint.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
How lived experiences of illness trajectories, burdens of treatment, and social inequalities shape service user and caregiver participation in health and social care: a theory-informed qualitative evidence synthesis.Health Soc Care Deliv Res. 2025 Jun;13(24):1-120. doi: 10.3310/HGTQ8159. Health Soc Care Deliv Res. 2025. PMID: 40548558
-
Rhipicephalus microplus voraxin-alpha contains B-cell epitopes that reduce ticks' biological fitness in immunized cattle.Ticks Tick Borne Dis. 2025 Jul;16(4):102516. doi: 10.1016/j.ttbdis.2025.102516. Epub 2025 Jul 8. Ticks Tick Borne Dis. 2025. PMID: 40633457
References
Publication types
LinkOut - more resources
Full Text Sources
