This is a preprint.
Preventing vision loss in a mouse model of Leber Congenital Amaurosis by engineered tRNA
- PMID: 40672310
- PMCID: PMC12265521
- DOI: 10.1101/2025.07.10.660754
Preventing vision loss in a mouse model of Leber Congenital Amaurosis by engineered tRNA
Abstract
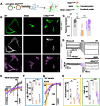
Premature termination codons (PTCs) are associated with rare genetic disorders. Inducing targeted read-through of these 'nonsense mutations' presents a potential therapeutic strategy for modifying disease outcomes. We previously reported that one such PTC, W53X, in the KCNJ13 gene causes blindness and Leber congenital amaurosis type-16 (LCA-16) due to loss of function of the inwardly rectifying potassium channel 7.1 (Kir7.1). Here, we present the proof of concept of a therapeutic approach based on anticodon-engineered transfer RNA (ACE-tRNA). The ACE-tRNA encodes the amino acid tryptophan (Trp) and suppresses the W53X PTC, restoring full-length protein expression. We used helper-dependent adenovirus (HDAd) to deliver the ACE-tRNATrp.UAG (tRNATrp.UAG) and rescue Kir7.1 function and physiology in patient-specific human induced pluripotent stem cell-derived retinal pigment epithelium (hiPSC-RPE) cells. Furthermore, in a W53X mouse model of LCA16, HDAd delivery of tRNATrp.UAG resulted in durable restoration of vision as measured by retinography. This study provides the first example of the therapeutic application of ACE-tRNA for treating an inherited form of blindness.
Keywords: Anticodon engineered tRNA; Kir7.1; Leber Congenital Amaurosis; RPE; electrophysiology; gene therapy; iPSC.
Figures



References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
