This is a preprint.
Avoidance engages dopaminergic punishment in Drosophila
- PMID: 40672319
- PMCID: PMC12265729
- DOI: 10.1101/2025.07.07.663268
Avoidance engages dopaminergic punishment in Drosophila
Abstract
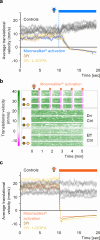
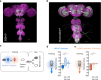
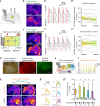
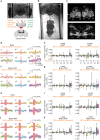
It was classically suggested that behaviour can cause emotions (Darwin 1872). For example, smiling can make us feel happier, and in rodents the induced patterns of cardiac activity and breathing that are indicative of fear can in turn evoke it (Coles et al. 2022, Hsueh et al. 2023, Jhang et al. 2024). However, the adaptive significance of such feedback is unclear. We show that inducing backward movement, an element of avoidance behaviour in Drosophila, engages negative valence signals in these animals, and reveal the neuronal mechanisms and adaptive significance of this effect. We develop a paradigm with odours as conditioned stimuli paired with optogenetically induced backward movement instead of a punishing unconditioned stimulus, and combined these experiments with pharmacology, high-resolution video tracking, functional imaging, connectome analyses, and modelling. Our results show that backward movement engages dopaminergic punishment neurons and supports aversive memories. Such avoidance-to-punishment feedback counterbalances extinction learning and maintains learned avoidance, reducing the risk of further punishment. This can explain the long-standing "avoidance paradox", the observation that avoidance adaptively persists even when it is successful and no punishment is received (Bolles 1972). Our results provide a neurobiologically grounded argument for an integrated view of behaviour organization and valence processing.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures


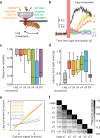

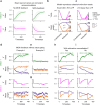






References
-
- Bidaye S. S., Machacek C., Wu Y. & Dickson B. J. Neuronal control of Drosophila walking direction. Science 344, 97–101 (2014). - PubMed
References Methods section
Publication types
LinkOut - more resources
Full Text Sources
