Combining Intramuscular and Intranasal Immunization With the MF59-Adjuvanted Respiratory Syncytial Virus Pre-Fusion Protein Subunit Vaccine Induces Potent Humoral and Cellular Immune Responses in Mice
- PMID: 40672433
- PMCID: PMC12264085
- DOI: 10.1002/mco2.70301
Combining Intramuscular and Intranasal Immunization With the MF59-Adjuvanted Respiratory Syncytial Virus Pre-Fusion Protein Subunit Vaccine Induces Potent Humoral and Cellular Immune Responses in Mice
Abstract
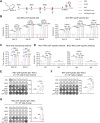
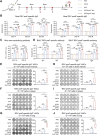
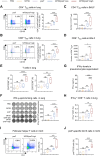
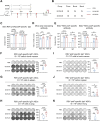
Respiratory syncytial virus (RSV) ranks as the second leading cause of infant death globally and a significant contributor to morbidity and mortality among adults over 60 years old. The development of effective RSV vaccines and immunoprophylaxis remains a key focus. In our research, we formulated a protein-based vaccine known as MF59/preF, which combines the RSV pre-fusion (preF) antigen with an MF59-like oil-in-water adjuvant. Intramuscular (IM) or intranasal (IN) immunization of the MF59-adjuvanted preF protein vaccine elicited robust immune responses and neutralizing antibodies against both RSV A2 and RSV B strains, with the IM showing a particularly pronounced effect. Notably, IN immunization with MF59/preF demonstrated superior mucosal immunity, characterized by elevated levels of IgA antibodies and an increased frequency of tissue-resident memory T (TRM) cells locally. More importantly, the combined IM and IN delivery of the MF59/preF vaccine synergistically enhanced antigen-specific humoral and cellular immune responses at both systemic and mucosal sites. Our study highlights the crucial impact of the route of administration and adjuvanted-protein subunit vaccines on triggering strong humoral and cellular immunity in mice.
Keywords: MF59 adjuvant; RSV pre‐fusion (preF) protein; intranasal immunization; mucosal immunity; respiratory syncytial virus (RSV).
© 2025 The Author(s). MedComm published by Sichuan International Medical Exchange & Promotion Association (SCIMEA) and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Wang X., Li Y., Shi T., et al., “Global Disease Burden of and Risk Factors for Acute Lower Respiratory Infections Caused by Respiratory Syncytial Virus in Preterm Infants and Young Children in 2019: A Systematic Review and Meta‐Analysis of Aggregated and Individual Participant Data,” Lancet 403, no. 10433 (2024): 1241–1253. - PubMed
-
- Rago A. R. P., D'Arrigo S. F., Osmani M., Espinosa C. M., Torres C. M., “Respiratory Syncytial Virus: Epidemiology, Burden of Disease, and Clinical Update,” Advances in Pediatrics 71, no. 1 (2024): 107–118. - PubMed
-
- Ruckwardt T. J., Morabito K. M., Graham B. S., “Immunological Lessons From Respiratory Syncytial Virus Vaccine Development,” Immunity 51, no. 3 (2019): 429–442. - PubMed
-
- Dalziel S. R., Haskell L., O'Brien S., et al., “Bronchiolitis,” Lancet 400, no. 10349 (2022): 392–406. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
