The neurological pathology of peroxisomal ACBD5 deficiency - lessons from patients and mouse models
- PMID: 40672445
- PMCID: PMC12263615
- DOI: 10.3389/fnmol.2025.1602343
The neurological pathology of peroxisomal ACBD5 deficiency - lessons from patients and mouse models
Abstract
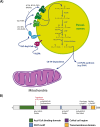
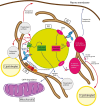
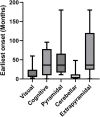
The absence or dysfunction of the peroxisomal membrane protein Acyl-CoA Binding Domain-Containing Protein 5 (ACBD5) is the cause of the most recently discovered peroxisomal disorder "Retinal Dystrophy with Leukodystrophy" (RDLKD). ACBD5 is a tail-anchored protein, anchored by its C-terminus into the peroxisomal membrane; hence, the bulk of its amino acid sequence faces the cytosol. With respect to ACBD5's molecular functions, RDLKD is unique since it is not only an accessory protein for the import of very-long-chain fatty acids (VLCFAs) into peroxisomes but also the first identified peroxisomal tethering protein facilitating membrane contacts with the endoplasmic reticulum (ER). Consequently, RDLKD is neither a peroxisomal biogenesis disorder nor single enzyme deficiency, since a deficiency in ACBD5 likely affects several aspects of peroxisomal function including VLCFA degradation, ether lipid synthesis, docosahexaenoic acid synthesis but also the transfer of membrane lipids from the ER to peroxisomes. Hence, RDLKD appears to be a multifactorial disorder leading to a mosaic pathology, combining symptoms caused by the disruption of several pathways. In this review, we will highlight recent findings obtained from case reports of RDLKD patients as well as insights from ACBD5-deficient mouse models to better understand its complex retinal and brain pathology. Moreover, we will discuss the possible contribution of the different dysregulated metabolites in the neurological pathogenesis of this latest peroxisomal disorder.
Keywords: ACBD5; RDLKD; VAP; fatty acid metabolism; membrane contact sites; peroxisomes.
Copyright © 2025 Dawes, Haberlander, Islinger and Schrader.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Al Shamsi B., Ganesh A., Harikrishna B., Al Zuhaibi S., Markovic I., Mansy A., et al. (2025). Retinal dystrophy and leukodystrophy caused by ACBD5 deficiency in five Omani patients: A case series. Oman. Med. J. 39. 10.5001/omj.2025.34 - DOI
Publication types
LinkOut - more resources
Full Text Sources

