This is a preprint.
Dominant negative ATP5F1A variants disrupt oxidative phosphorylation causing neurological disorders
- PMID: 40672495
- PMCID: PMC12265762
- DOI: 10.1101/2025.07.08.25330848
Dominant negative ATP5F1A variants disrupt oxidative phosphorylation causing neurological disorders
Update in
-
Dominant negative ATP5F1A variants disrupt oxidative phosphorylation causing neurological disorders.EMBO Mol Med. 2025 Oct;17(10):2562-2585. doi: 10.1038/s44321-025-00290-8. Epub 2025 Aug 26. EMBO Mol Med. 2025. PMID: 40859057 Free PMC article.
Abstract
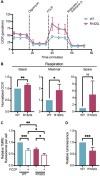
ATP5F1A encodes the α-subunit of complex V of the respiratory chain, which is responsible for mitochondrial ATP synthesis. We describe 6 probands with heterozygous de novo missense ATP5F1A variants that presented with developmental delay, intellectual disability, and movement disorders. Functional evaluation in C. elegans revealed that all variants tested were damaging to gene function via a dominant negative genetic mechanism. Biochemical and proteomics studies showed a marked reduction in complex V abundance and activity in proband-derived blood cells and fibroblasts. Mitochondrial physiology studies in fibroblasts revealed increased oxygen consumption, yet decreased mitochondrial membrane potential and ATP levels indicative of uncoupled oxidative phosphorylation as a pathophysiologic mechanism. Our findings contrast functionally and clinically with the previously reported ATP5F1A variant, p.Arg207His, suggesting a distinct pathological mechanism. This study therefore expands the phenotypic and genotypic spectrum of ATP5F1A-associated conditions and highlights how functional studies can provide understanding of the genetic, molecular, and cellular mechanisms of ATP5F1A variants of uncertain significance. With 12 heterozygous individuals now reported, ATP5F1A is the most frequent nuclear genome cause of complex V deficiency.
Keywords: ATP synthase; ATP5F1A; C. elegans; Complex V; developmental delay; dystonia; intellectual disability; mitochondriopathy; oxidative phosphorylation; variant.
Figures






References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
