The advance on pathophysiological mechanisms of type 2 chronic rhinosinusitis with nasal polyposis
- PMID: 40672703
- PMCID: PMC12263562
- DOI: 10.3389/falgy.2025.1599797
The advance on pathophysiological mechanisms of type 2 chronic rhinosinusitis with nasal polyposis
Abstract
Purpose: This review aims to explore the pathophysiological mechanisms and emerging therapies for type 2 chronic rhinosinusitis with nasal polyps (CRSwNP), driven primarily by type 2 inflammation.
Search methods: A comprehensive search of relevant literature was performed in databases including PubMed, Web of Science, and Scopus, using keywords such as "chronic rhinosinusitis with nasal polyps," "type 2 inflammation," "Th2 cells," "ILC2s," "epithelial barrier dysfunction," and "biologics". The search was limited to articles published from January 2010 to February 2025.
Search results: A total of 200 articles were initially retrieved. After screening based on relevance and quality, 163 articles were selected for this review. These included 109 basic research papers, 30 clinical studies, and 24 review articles.
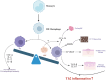
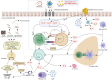
Conclusions: Type 2 CRSwNP pathogenesis involves Th2/ILC2-IL-4/IL-13 synergy, driving eosinophilic inflammation and tissue remodeling via a self-amplifying loop. Programmed cell death protein 1 and programmed death-ligand 1 dysregulation intensifies Th2 responses. Epithelial barrier defects (via disrupted junctions and ciliary defects) and epithelial-mesenchymal transition facilitate pathogen invasion and stromal changes. M2 macrophages amplify inflammation via CCL-24 and Staphylococcus aureus synergy, sustaining biofilm persistence. Targeted biologics-dupilumab (IL-4Rα inhibitor) reduces polyp burden and restores smell, while mepolizumab (anti-IL-5) and omalizumab (anti-IgE) address specific endotypes. Despite therapeutic advances, biologics require real-world validation for long-term safety and cost-effectiveness.
Keywords: biologics; chronic rhinosinusitis with nasal polyposis (CRSwNP); epithelial barrier dysfunction; type 2 T helper cells (Th2); type 2 innate lymphoid cells (ILC2s).
© 2025 Yang, Guo, Wang, Jiang, Chen and Gu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Improvement in Smell Using Monoclonal Antibodies Among Patients With Chronic Rhinosinusitis With Nasal Polyps: A Systematic Review.J Investig Allergol Clin Immunol. 2023 Dec 14;33(6):419-430. doi: 10.18176/jiaci.0939. Epub 2023 Sep 4. J Investig Allergol Clin Immunol. 2023. PMID: 37669083
-
Equivalent healthcare resource use following either a long-acting steroid-eluting implant or repeat endoscopic surgery for chronic rhinosinusitis patients with nasal polyp recurrence: a real-world evidence study.Curr Med Res Opin. 2025 May;41(5):767-777. doi: 10.1080/03007995.2025.2513955. Epub 2025 Jun 11. Curr Med Res Opin. 2025. PMID: 40478176
-
Which Is the Best Biologic for Nasal Polyps: Dupilumab, Omalizumab, or Mepolizumab? A Network Meta-Analysis.Int Arch Allergy Immunol. 2022;183(3):279-288. doi: 10.1159/000519228. Epub 2021 Oct 4. Int Arch Allergy Immunol. 2022. PMID: 34607329
-
Efficacy of different biologics for treating chronic rhinosinusitis with nasal polyps: a network meta-analysis.Eur Arch Otorhinolaryngol. 2025 Feb;282(2):559-569. doi: 10.1007/s00405-024-08903-7. Epub 2024 Aug 26. Eur Arch Otorhinolaryngol. 2025. PMID: 39187717
-
Systemic and topical antibiotics for chronic rhinosinusitis.Cochrane Database Syst Rev. 2016 Apr 26;4(4):CD011994. doi: 10.1002/14651858.CD011994.pub2. Cochrane Database Syst Rev. 2016. PMID: 27113482 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

