Pharmacokinetics and outcome of high-dose melphalan followed by autologous stem cell transplantation in dialysis-dependent patients with multiple myeloma
- PMID: 40672810
- PMCID: PMC12264629
- DOI: 10.1016/j.lrr.2025.100522
Pharmacokinetics and outcome of high-dose melphalan followed by autologous stem cell transplantation in dialysis-dependent patients with multiple myeloma
Abstract
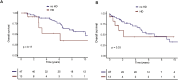
High-dose melphalan followed by autologous stem cell transplantation (ASCT) remains the standard of care for fit patients with multiple myeloma (MM). However, individuals who are dependent on hemodialysis are frequently excluded from ASCT. Recommendations on chemotherapy dosing and hemodialysis scheduling vary in literature and definite conclusions are impeded by the heterogeneity of cohorts. We aimed to evaluate the safety and efficacy of ASCT in patients with MM and end-stage renal disease and to examine the pharmacokinetics of melphalan on a fixed schedule of melphalan infusion and hemodialysis. The outcome of 13 patients undergoing ASCT while being on hemodialysis between 2000 and 2022 was retrospectively analysed and compared to matched hemodialysis-independent patients. Melphalan plasma concentrations were measured in 4 hemodialysis-dependent and 5 independent patients. Plasma concentrations of hemodialysis-dependent patients were comparable to hemodialysis-independent patients with a 6-hour interval between melphalan infusion and hemodialysis (p = 0.9). The rate of immediate side effects of high-dose melphalan was significantly higher in 13 dialysis-dependent patients compared to 47 matched controls despite not having prolonged neutropenia (p = 0.9). Overall survival both from d0 of ASCT and diagnosis was comparable (p = 0.33 and p = 0.17, respectively). Thus, adopting the proposed schedule and management of immediate side effects make ASCT a safe option for myeloma patients with end-stage renal disease.
Keywords: Autologous stem cell transplantation; Hemodialysis; High-dose melphalan; Multiple myeloma.
© 2025 The Author(s).
Conflict of interest statement
BMWS received lecture fees and honoraria from ADVITOS, Amgen, Bayer Vital, Berlin Chemie-Menarini, CytoSorbents, Daichii Sankyo, Miltenyi, Pocard, not related to this article. AB has participated in advisory boards from BMS, Janssen, GSK, Takeda and Sanofi and received honoraria or travel support from BMS, Janssen, GSK, Sanofi, Amgen and AstraZeneca. FHH served as an advisor for Novartis, CTI, Celgene/BMS, Janssen, Abbvie, GSK, Merck and AOP and received research funding from Novartis, Celgene/BMS and CTI, not related to this article.
Figures

Similar articles
-
First-line tandem high-dose chemotherapy and autologous stem cell transplantation versus single high-dose chemotherapy and autologous stem cell transplantation in multiple myeloma, a systematic review of controlled studies.Cochrane Database Syst Rev. 2012 Oct 17;10(10):CD004626. doi: 10.1002/14651858.CD004626.pub3. Cochrane Database Syst Rev. 2012. PMID: 23076906 Free PMC article.
-
High-dose therapy with autologous stem cell transplantation versus chemotherapy or immuno-chemotherapy for follicular lymphoma in adults.Cochrane Database Syst Rev. 2012 Jan 18;1(1):CD007678. doi: 10.1002/14651858.CD007678.pub2. Cochrane Database Syst Rev. 2012. PMID: 22258971 Free PMC article.
-
The clinical effectiveness and cost-effectiveness of bortezomib and thalidomide in combination regimens with an alkylating agent and a corticosteroid for the first-line treatment of multiple myeloma: a systematic review and economic evaluation.Health Technol Assess. 2011 Dec;15(41):1-204. doi: 10.3310/hta15410. Health Technol Assess. 2011. PMID: 22146234 Free PMC article.
-
The Impact of Melphalan Conditioning and CD34 + Cell Dose and Schedule on Post-Transplant Outcomes in AL Amyloidosis.Am J Hematol. 2025 Jul;100(7):1141-1151. doi: 10.1002/ajh.27685. Epub 2025 Apr 11. Am J Hematol. 2025. PMID: 40214172
-
High-dose chemotherapy followed by autologous stem cell transplantation for patients with relapsed/refractory Hodgkin lymphoma.Cochrane Database Syst Rev. 2013 Jun 20;2013(6):CD009411. doi: 10.1002/14651858.CD009411.pub2. Cochrane Database Syst Rev. 2013. PMID: 23784872 Free PMC article.
References
-
- Attal M., Harousseau J., Stoppa A., et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. N. Engl. J. Med. 1996;335(2):91–97. doi: 10.1056/NEJM199607113350204. http://content.nejm.org/cgi/content/abstract/335/2/91 - DOI - PubMed
-
- Child J.A., Morgan G.J., Davies F.E., et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N. Engl. J. Med. 2003;348(19):1875–1883. doi: 10.1056/NEJMoa022340. http://content.nejm.org/cgi/content/abstract/348/19/1875 - DOI - PubMed
-
- Richardson P.G., Jacobus S.J., Weller E.A., et al. Triplet therapy, transplantation, and maintenance until progression in myeloma. N. Engl. J. Med. 2022;387(2):132–147. doi: 10.1056/NEJMoa2204925. https://nejm.org/doi/full/10.1056/NEJMoa 2204925. - DOI - DOI - PMC - PubMed
-
- Cavo M., Gay F., Beksac M., et al. Upfront autologous hematopoietic stem-cell transplantation improves overall survival in comparison with bortezomib-based intensification therapy in newly diagnosed multiple myeloma: long-term follow-up analysis of the randomized phase 3 EMN02/HO95 study. Blood. 2020;136(Supplement 1):37–38. doi: 10.1182/blood-2020-137575. - DOI
-
- Dimopoulos M.A., Terpos E., Comenzo R.L., et al. Renal impairment in patients with multiple myeloma: a consensus statement on behalf of the international myeloma working group. JCO. 2010;28(33):4976–4984. doi: 10.1200/JCO.2010.30.8791. http://jco.ascopubs.org/content/28/33/4976.abstract - DOI - PubMed
LinkOut - more resources
Full Text Sources

