An Examination into the Safety and Efficacy of Khapregesic®, a Khaya Senegalensis Preparation, on Women Experiencing Menstrual Pain and Menstrual Distress: A Randomized, Double-Blind, Placebo-Controlled Trial
- PMID: 40672875
- PMCID: PMC12266064
- DOI: 10.2147/IJWH.S521349
An Examination into the Safety and Efficacy of Khapregesic®, a Khaya Senegalensis Preparation, on Women Experiencing Menstrual Pain and Menstrual Distress: A Randomized, Double-Blind, Placebo-Controlled Trial
Abstract
Purpose: In traditional medicine, Khaya senegalensis has been used to treat menstrual pain, dysmenorrhea, and digestive pain and discomfort. However, there are no human clinical trials examining its safety and efficacy for the treatment of menstrual distress. Therefore, the purpose of this two-arm, parallel-group, randomized, double-blind, placebo-controlled trial was to examine the safety and efficacy of supplementation with a Khaya senegalensis preparation (Khapregesic®) on menstrual pain and menstrual distress in menstruating women.
Methods: Eighty-four women experiencing menstrual pain and distress were supplemented 3g daily with this Khaya senegalensis preparation or a placebo for one menstrual cycle. Changes in menstrual pain and other symptoms of menstrual distress were examined through daily ratings and validated self-report questionnaires. Moreover, changes in the use of rescue medications, C-reactive protein, and safety blood measures were examined.
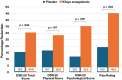
Results: Compared to the placebo, this Khaya senegalensis preparation was associated with greater reductions in daily menstrual pain ratings (p=0.033) and reductions in overall menstrual distress (p=0.042). Improvements in emotional wellbeing were also identified, along with reductions in the use of rescue medications, although this latter finding requires confirmation in future trials. No changes in C-reactive protein were identified. This Khaya senegalensis preparation was well-tolerated and there were no significant changes in safety blood markers.
Conclusion: This study provides evidence supporting the safety and efficacy of a Khaya senegalensis preparation on menstrual pain and menstrual distress in women. Further investigations will be important to confirm and expand on the current findings and to help identify its potential mechanisms of action.
Trial registration: ANZCTR, ACTRN12624000731594p. Registered 14 June 2024, https://www.anzctr.org.au/ACTRN12624000731594p.aspx.
Keywords: clinical trial; dysmenorrhea; herbal medicine; menstrual disturbances.
© 2025 Lopresti et al.
Conflict of interest statement
ALL is the managing director of Clinical Research Australia, a contract research organization that receives research funding from nutraceutical companies. ALL has also received presentation honoraria from nutraceutical companies. SJS is an employee of Clinical Research Australia. FRF is the head of research, co-founder, and CEO of Bioactive Natural Health Pty Ltd, the study funder and the manufacturer of the investigational product examined in this study. The authors report no other conflicts of interest in this work.
Figures
Similar articles
-
Herbal and dietary therapies for primary and secondary dysmenorrhoea.Cochrane Database Syst Rev. 2001;(3):CD002124. doi: 10.1002/14651858.CD002124. Cochrane Database Syst Rev. 2001. Update in: Cochrane Database Syst Rev. 2016 Mar 22;3:CD002124. doi: 10.1002/14651858.CD002124.pub2. PMID: 11687013 Updated.
-
Treatments for seizures in catamenial (menstrual-related) epilepsy.Cochrane Database Syst Rev. 2021 Sep 16;9(9):CD013225. doi: 10.1002/14651858.CD013225.pub3. Cochrane Database Syst Rev. 2021. PMID: 34528245 Free PMC article.
-
Interventions to prevent or treat heavy menstrual bleeding or pain associated with intrauterine-device use.Cochrane Database Syst Rev. 2022 Aug 26;8(8):CD006034. doi: 10.1002/14651858.CD006034.pub3. Cochrane Database Syst Rev. 2022. PMID: 36017945 Free PMC article.
-
Progesterone or progestogen-releasing intrauterine systems for heavy menstrual bleeding.Cochrane Database Syst Rev. 2005 Oct 19;(4):CD002126. doi: 10.1002/14651858.CD002126.pub2. Cochrane Database Syst Rev. 2005. Update in: Cochrane Database Syst Rev. 2015 Apr 30;(4):CD002126. doi: 10.1002/14651858.CD002126.pub3. PMID: 16235297 Updated.
-
Pre-operative endometrial thinning agents before endometrial destruction for heavy menstrual bleeding.Cochrane Database Syst Rev. 2013 Nov 15;2013(11):CD010241. doi: 10.1002/14651858.CD010241.pub2. Cochrane Database Syst Rev. 2013. PMID: 24234875 Free PMC article.
References
-
- RIcha SY, Minaskshi S. Menstrual distress and its impact on quality of life of adolescents and middle-aged women. Clin Case Reports Int. 2024;8:1644.
Publication types
LinkOut - more resources
Full Text Sources
Research Materials