A retrospective real-world single-arm study evaluating the efficacy and safety of neoadjuvant chemotherapy in patients with selected limited-stage small-cell lung cancer
- PMID: 40673100
- PMCID: PMC12261241
- DOI: 10.21037/tlcr-2025-209
A retrospective real-world single-arm study evaluating the efficacy and safety of neoadjuvant chemotherapy in patients with selected limited-stage small-cell lung cancer
Abstract
Background: With the advancement of surgical techniques and the introduction of neoadjuvant therapies, the risk of recurrence or distant metastases has been significantly decreased for non-small-cell lung cancer (non-SCLC) after surgery. In recent years, the application of these advanced techniques and therapies in SCLC has also shown promise. This study aims to explore the efficacy and safety of neoadjuvant chemotherapy combined with surgery in selected limited-stage SCLC (LS-SCLC).
Methods: In this retrospective, single-arm clinical trial, we conducted a thorough review of electronic medical records from the Shanghai Pulmonary Hospital between December 2015 and December 2022. Patients with a pathological diagnosis of SCLC who underwent neoadjuvant chemotherapy followed by radical surgery were enrolled. Baseline demographic and clinical characteristics, specifics of neoadjuvant therapy and surgery, survival outcomes, and safety profiles of included patients were systematically collected and analyzed.
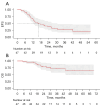
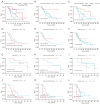
Results: A total of 47 patients [7 (14.89%) females and 40 (85.11%) males; median age 61.00 years, interquartile range (IQR), 55.50-67.50 years] were enrolled. The disease control rate was 100%, with an objective response rate of 70.21% and a downstaging rate of 65.9%. The percentage of patients with a complete pathological response (CPR) and major pathological response (MPR) was 10.64% (5/47) and 12.77% (6/47, excluding CPR), respectively. In subgroups stratified by baseline demographic and clinical characteristics, the MPR rate showed no significant differences, yet a trend toward higher MPR was observed among smoking patients. At the data cutoff (October 2, 2024), the median follow-up period was 35.367 months [IQR, 26.367 months-not reached (NR)]. The median event-free survival (EFS) was 16.27 months [95% confidence interval (CI): 12.20-30.53] and the median overall survival (OS) was NR, with 2-, 3-, and 4-year survival rates of 79.96% (95% CI: 68.36-93.52%), 71.39% (95% CI: 57.12-89.22%), and 64.90% (95% CI: 48.52-86.82%), respectively. The stratified analysis revealed that patients achieving an MPR and those undergoing postoperative adjuvant radiotherapy exhibited longer EFS and OS. Treatment-related adverse events of grade 3-4 were observed in 21.28% of patients, with the most frequent occurrences being a decrease in neutrophil count (12.77%), followed by a decrease in platelet count (8.51%), and a decrease in white blood cell count (4.26%).
Conclusions: Neoadjuvant chemotherapy combined with surgery could be a potential treatment strategy for LS-SCLC, with a high proportion of patients achieving an MPR, and manageable safety profile, that did not compromise surgical resection. Further prospective clinical trials are warranted to delineate the benefits of neoadjuvant chemotherapy and optimize LS-SCLC treatment.
Keywords: Small-cell lung cancer (SCLC); major pathological response (MPR); neoadjuvant chemotherapy; surgery.
Copyright © 2025 AME Publishing Company. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2025-209/coif). The authors have no conflicts of interest to declare.
Figures



Similar articles
-
Postoperative adjuvant chemotherapy in rectal cancer operated for cure.Cochrane Database Syst Rev. 2012 Mar 14;2012(3):CD004078. doi: 10.1002/14651858.CD004078.pub2. Cochrane Database Syst Rev. 2012. PMID: 22419291 Free PMC article.
-
Hysterectomy with radiotherapy or chemotherapy or both for women with locally advanced cervical cancer.Cochrane Database Syst Rev. 2015 Apr 7;(4):CD010260. doi: 10.1002/14651858.CD010260.pub2. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2022 Aug 22;8:CD010260. doi: 10.1002/14651858.CD010260.pub3. PMID: 25847525 Updated.
-
The effectiveness and cost-effectiveness of carmustine implants and temozolomide for the treatment of newly diagnosed high-grade glioma: a systematic review and economic evaluation.Health Technol Assess. 2007 Nov;11(45):iii-iv, ix-221. doi: 10.3310/hta11450. Health Technol Assess. 2007. PMID: 17999840
-
Interim analysis of the efficiency and safety of neoadjuvant PD-1 inhibitor (sintilimab) combined with chemotherapy (nab-paclitaxel and carboplatin) in potentially resectable stage IIIA/IIIB non-small cell lung cancer: a single-arm, phase 2 trial.J Cancer Res Clin Oncol. 2023 Feb;149(2):819-831. doi: 10.1007/s00432-021-03896-w. Epub 2022 Feb 22. J Cancer Res Clin Oncol. 2023. PMID: 35192053 Free PMC article. Clinical Trial.
-
Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924
References
LinkOut - more resources
Full Text Sources
