Esketamine Optimized the Efficacy of Dexmedetomidine in Treating Sleep Disorders with Comorbid Depression
- PMID: 40673111
- PMCID: PMC12266076
- DOI: 10.2147/NDT.S530265
Esketamine Optimized the Efficacy of Dexmedetomidine in Treating Sleep Disorders with Comorbid Depression
Abstract
Purpose: Although Dexmedetomidine (DEX) can induce sleep that resembles natural sleep, it has demonstrated limited efficacy in patients with comorbid insomnia and depression. On the other hand, Esketamine (ESK) has shown a potent antidepressant effect. Herein, we aimed to establish whether esketamine could enhance the therapeutic efficacy of DEX in treating patients with comorbid insomnia and depression.
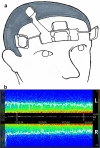
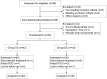
Methods: We recruited 84 patients with comorbid insomnia and depression who were randomized into two groups for a 1-month follow-up study: the DE group (receiving dexmedetomidine and esketamine) and the DS group (receiving dexmedetomidine and saline). Outcome measures included polysomnographic monitoring (PSG), Montgomery-Åsberg Depression Rating Scale (MADRS), Pittsburgh Sleep Quality Index (PSQI), Sleep Numeric Rating Scale (SNRS), and serum brain-derived neurotrophic factor (BDNF) concentrations. The primary outcome was a comparison of PSG parameters recorded at baseline (D0) and on treatment day 3 (D3).
Results: After 3 days of treatment, patients in DE group had a significant increase in total sleep duration, duration and proportion of N3 sleep (P < 0.05), a significant decrease in proportion of N2 sleep and proportion of REM sleep (P < 0.05), and a significant decrease in depression score and sleep numeric rating scale score (P < 0.05), as compared with DS group. Improvements in sleep were associated with improvements in MADRS score and increases in BDNF. Oral dryness was the most frequent adverse event (AE).
Conclusion: When combined with ESK, DEX improved patients' depression scores, further extended total sleep time, increased the N3 sleep proportion, and enhanced deep sleep continuity, with few AEs.
Keywords: depression; dexmedetomidine; esketamine; insomnia; polysomnography.
© 2025 Ding et al.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

