Efficient biodegradation and upcycling of polyethylene terephthalate mediated by cell-factories
- PMID: 40673144
- PMCID: PMC12263691
- DOI: 10.3389/fmicb.2025.1599470
Efficient biodegradation and upcycling of polyethylene terephthalate mediated by cell-factories
Abstract
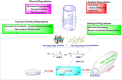
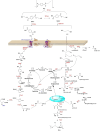
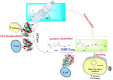
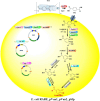
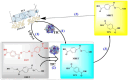
The pervasive accumulation of polyethylene terephthalate (PET) waste has emerged as a critical ecological crisis, which is mainly driven by its recalcitrance to natural degradation and widespread contamination of terrestrial and aquatic ecosystems. In response to this challenge, microbial-mediated PET biodegradation has garnered significant scientific attentions as a sustainable remediation strategy, harnessing the enzymatic cascades of specialized microorganisms to depolymerize PET into bio-assimilable monomers such as terephthalic acid (TPA) and ethylene glycol (EG). In this review, we summarize the extracellular process of PET biodegradation, including microbial attachment, colonization, and direct depolymerization, as well as the metabolic pathways of PET monomers. Strategies for developing PET-degrading chassis cells are also discussed, such as cell surface display, metabolic pathway optimization, and rational design of enzyme-PET interfaces. Microbial-enzyme consortia and molecular engineering of photosynthetic microorganisms also contribute to PET degradation. Although significant progress has been made, challenges remain in enzyme stability, metabolic bottlenecks, industrial scalability, and environmental adaptation. Overall, microbial and enzymatic strategies show great potentials in addressing PET pollution, and future interdisciplinary efforts are needed to overcome these challenges and achieve a sustainable circular plastic economy.
Keywords: PET recycling; cell factory; metabolic engineering; microbial degradation; polyethylene terephthalate; synthetic biology.
Copyright © 2025 Liu, Wang, Liu, Xu and Pan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures










Similar articles
-
Engineered Halomonas sp. Y3 enables highly efficient upcycling of poly(ethylene terephthalate) to polyhydroxyalkanoates.Bioresour Technol. 2025 Nov;436:133039. doi: 10.1016/j.biortech.2025.133039. Epub 2025 Jul 26. Bioresour Technol. 2025. PMID: 40721073
-
Bio-upcycling PET waste: Advances in enzymatic hydrolysis and biosynthesis of value-added products.Biotechnol Adv. 2025 Aug 9;84:108685. doi: 10.1016/j.biotechadv.2025.108685. Online ahead of print. Biotechnol Adv. 2025. PMID: 40789538 Review.
-
Exploring the biodegradation of PET in mangrove soil and its intermediates by enriched bacterial consortia.Environ Technol. 2025 Jul 9:1-23. doi: 10.1080/09593330.2025.2521762. Online ahead of print. Environ Technol. 2025. PMID: 40629992
-
Efficient secretion of a plastic degrading enzyme from the green algae Chlamydomonas reinhardtii.Sci Rep. 2025 Jul 9;15(1):24690. doi: 10.1038/s41598-025-09100-0. Sci Rep. 2025. PMID: 40634484 Free PMC article.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
References
-
- Almeida E., Carrillo Rincón A., Jackson S., Dobson A. (2019). In silico screening and heterologous expression of a polyethylene terephthalate hydrolase (PETase)-like enzyme (SM14est) with polycaprolactone (PCL)-degrading activity, from the marine sponge-derived strain Streptomyces sp. SM14. Front. Microbiol. 10:2187. 10.3389/fmicb.2019.02187 - DOI - PMC - PubMed
-
- Arijeniwa V., Akinsemolu A., Chukwugozie D., Onawo U., Ochulor C., Nwauzoma U., et al. (2024). Closing the loop: A framework for tackling single-use plastic waste in the food and beverage industry through circular economy–a review. J. Environ. Manag. 359:120816. 10.1016/j.jenvman.2024.120816 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

