Exploring the additive antibacterial potential of Cinnamomum cassia volatile oil and imipenem against Acinetobacter baumannii: a multi-omics investigation
- PMID: 40673148
- PMCID: PMC12263672
- DOI: 10.3389/fmicb.2025.1578322
Exploring the additive antibacterial potential of Cinnamomum cassia volatile oil and imipenem against Acinetobacter baumannii: a multi-omics investigation
Abstract
Introduction: Acinetobacter baumannii has been identified as a critical pathogen, and new antibiotics are urgently needed. Volatile oils, which function as natural antibacterial agents, may provide an effective means of inhibiting A. baumannii. However, the antibacterial activity and mechanism of the volatile oil derived from the dried bark of Cinnamomum cassia (CBV), as well as its additive effect when combined with imipenem (IPM) against A. baumannii, remain unclear.
Methods: CBV was extracted using the hydrodistillation method and characterized by gas chromatography-mass spectrometry (GC-MS) analysis. The minimum inhibitory concentrations (MICs) of CBV and IPM against A. baumannii were determined using the microdilution method. A checkerboard assay was performed to evaluate the additive effect of CBV (concentration range: 0-1 μL/mL) and IPM (concentration range: 0-256 μg/mL) against A. baumannii, with the fractional inhibitory concentration index (FICI) calculated. A time-kill curve analysis was performed to assess the additive effect of CBV (0.125 μL/mL) and IPM (4 μg/mL) against A. baumannii. Antibiofilm activity was evaluated using a crystal violet staining assay. Cell membrane integrity was assessed using SYTO 9/PI staining based on fluorescence color. Intracellular protein levels were quantified using a BCA kit according to the manufacturer's instructions. Scanning electron microscopy (SEM) was used to observe morphological changes in A. baumannii. Additionally, the antibacterial mechanism was elucidated through a combination of transcriptomic and proteomic analyses.
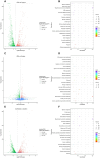
Results: An additive effect (FICI = 0.53) was observed when CBV and IPM were combined against A. baumannii, reducing the MIC of IPM from 256 μg/mL to 4 μg/mL. CBV and IPM inhibited biofilm formation, damaged the cell membrane, and induced intracellular protein leakage in A. baumannii. Compared to CBV or IPM alone, the combination group (at the dosage showing an additive effect) caused significantly greater damage to the cell membrane of A. baumannii. CBV and IPM also induced significant changes at both the transcriptomic and proteomic levels in A. baumannii. Functional analysis revealed that the differentially expressed genes (DEGs) and proteins (DEPs) were involved in multiple pathways. Both CBV and IPM contributed to the observed antibacterial activity. CBV primarily influenced the ribosome pathway, while IPM mainly influenced oxidative phosphorylation. In the combination treatment, the simultaneous targeting of the ribosome and oxidative phosphorylation pathways was identified as the key antibacterial mechanism.
Conclusion: This study demonstrated that the combination of CBV and IPM exhibits promising antimicrobial activity against A. baumannii, suggesting that CBV could serve as a potential natural candidate for the development of novel antibiotic agents. While the current findings establish a mechanistic foundation for CBV's antimicrobial effects, further research is necessary to facilitate its clinical translation. Specifically, formulation optimization studies are necessary to enhance the therapeutic viability of the CBV/IPM combination, and comprehensive in vivo investigations are crucial to validate the antibacterial efficacy and safety profile of CBV/IPM prior to clinical application.
Keywords: Acinetobacter baumannii; antibiotic agents; antimicrobial activity; imipenem; volatile oil of Cinnamomum cassia.
Copyright © 2025 Lu, Xu, Xue, Xie, Liu, Wang, Li and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Appaneal H. J., Lopes V. V., LaPlante K. L., Caffrey A. R. (2022). Treatment, clinical outcomes, and predictors of mortality among a national cohort of admitted patients with Acinetobacter baumannii infection. Antimicrob. Agents Chemother. 66:e01975-01921. doi: 10.1128/aac.01975-21, PMID: - DOI - PMC - PubMed
-
- Cai R., Liang Y., Yuan Y., Sheng Q., Wang Z., Yue T. (2024). Antimicrobial activity and mechanism of chitosan-coated epsilon-poly-lysine nanoliposomes against Alicyclobacillus spp. in apple juice. Food Control 164:110589. doi: 10.1016/j.foodcont.2024.110589 - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

