Circulating tumor DNA in cholangiocarcinoma: current clinical applications and future perspectives
- PMID: 40673275
- PMCID: PMC12263599
- DOI: 10.3389/fcell.2025.1616064
Circulating tumor DNA in cholangiocarcinoma: current clinical applications and future perspectives
Abstract
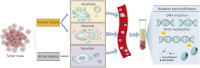
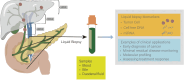
Cholangiocarcinoma is a highly heterogeneous malignant tumor, including intrahepatic cholangiocarcinoma, hepatoportal cholangiocarcinoma and distal cholangiocarcinoma. Its incidence is increasing worldwide and currently accounts for approximately 15% of all primary liver cancers and 3% of all gastrointestinal malignancies. There is a lack of early diagnostic methods for cholangiocarcinoma, and the overall treatment effect is poor, with a 5-year survival rate of less than 25%. New biomarkers are urgently needed in clinical practice to improve the current diagnosis and treatment status. Circulating tumor DNA (ctDNA) is DNA fragments released by tumor cells, which can show tumor-specific gene mutations (such as IDH1/2, FGFR2 fusion) and epigenetic modifications (such as abnormal methylation). With the rapid development of tumor liquid biopsy technology, ctDNA has been gradually applied in solid tumors such as lung cancer and colorectal cancer due to its high sensitivity and dynamic monitoring capabilities. This review systematically introduces ctDNA technology and its progress in early screening, early diagnosis, treatment response, and prognosis monitoring of cholangiocarcinoma. In addition, this review also summarizes the challenges and limitations of current ctDNA technology and analyzes future hot research directions.
Keywords: cholangiocarcinoma; circulating tumor DNA; liquid biopsy; prognosis monitoring; tumor-informed ctDNA.
Copyright © 2025 Wang, Li, Liang, Zhang, Li, Tian, Zhao, Jin, Cao and Lin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Can a Liquid Biopsy Detect Circulating Tumor DNA With Low-passage Whole-genome Sequencing in Patients With a Sarcoma? A Pilot Evaluation.Clin Orthop Relat Res. 2025 Jan 1;483(1):39-48. doi: 10.1097/CORR.0000000000003161. Epub 2024 Jun 21. Clin Orthop Relat Res. 2025. PMID: 38905450
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Exploring the utility of ctDNA testing in high-risk breast cancer patients in a community setting: case series.Ther Adv Med Oncol. 2025 Jul 8;17:17588359251351121. doi: 10.1177/17588359251351121. eCollection 2025. Ther Adv Med Oncol. 2025. PMID: 40656596 Free PMC article.
-
Biliary tract cancers: advances in diagnostic and management.Explor Target Antitumor Ther. 2025 Jun 23;6:1002328. doi: 10.37349/etat.2025.1002328. eCollection 2025. Explor Target Antitumor Ther. 2025. PMID: 40585845 Free PMC article. Review.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
References
-
- Abou-Alfa G. K., Macarulla T., Javle M. M., Kelley R. K., Lubner S. J., Adeva J., et al. (2020a). Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): a multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 21, 796–807. 10.1016/S1470-2045(20)30157-1 - DOI - PMC - PubMed
-
- Abou-Alfa G. K., Sahai V., Hollebecque A., Vaccaro G., Melisi D., Al-Rajabi R., et al. (2020b). Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol. 21, 671–684. 10.1016/S1470-2045(20)30109-1 - DOI - PMC - PubMed
-
- Anagnostou V., Velculescu V. E. (2024). Pushing the boundaries of liquid biopsies for early precision intervention. Cancer Discov. 14, 615–619. 10.1158/2159-8290.CD-24-0037 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

