Hippo/YAP signaling's multifaceted crosstalk in cancer
- PMID: 40673277
- PMCID: PMC12263953
- DOI: 10.3389/fcell.2025.1595362
Hippo/YAP signaling's multifaceted crosstalk in cancer
Abstract
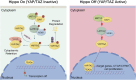
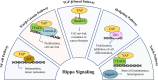
The Hippo/yes-associated protein (YAP) signaling is an evolutionarily conserved regulator in organ size control, which plays pivotal roles in cell proliferation, differentiation, apoptosis, and tissue regeneration. In cancer, dysregulation of Hippo/YAP signaling is typically recognized as one of the crucial drivers in tumorigenesis. However, beyond its canonical transcriptional targets, Hippo/YAP signaling engages in extensive crosstalk with multiple pathways to form an intricate regulatory network, thereby giving rise to its content-dependent influence on tumor initiation, progression and metastasis. This review focuses on the molecular mechanisms underlying the interplay between Hippo/YAP and pivotal signaling pathways such as nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), wingless-type (Wnt)/β-catenin signaling pathway, transforming growth factor-beta (TGF-β), Hedgehog, Notch and other signaling pathways, as well as their implications in cancer biology. Ultimately, exploiting these mechanisms may represent promising therapeutic strategies for cancer.
Keywords: Hippo/YAP signaling; cancer; crosstalk; signaling pathway; therapeutic target.
Copyright © 2025 Zhang, Wu, Ren, Chen, Ye, Chen, Fang, Wu and Zhao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Abou-Alfa G. K., Meyer T., Do R. K. G., Piha-Paul S. A., Light J. S., Sherrin S., et al. (2025). Neratinib alone or in combination with immune checkpoint inhibitors with or without mammalian target of rapamycin inhibitors in patients with fibrolamellar carcinoma. Liver Cancer 14 (1), 58–67. 10.1159/000540290 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

