[S1PR5 activation or overexpression enhances barrier function of mouse brain microvascular endothelial cells against OGD/R injury by modulating oxidative stress]
- PMID: 40673307
- PMCID: PMC12268917
- DOI: 10.12122/j.issn.1673-4254.2025.07.11
[S1PR5 activation or overexpression enhances barrier function of mouse brain microvascular endothelial cells against OGD/R injury by modulating oxidative stress]
Abstract
Objectives: To investigate the role of sphingosine-1-phosphate receptor 5 (S1PR5) in modulating barrier function of mouse brain microvascular endothelial cells with oxygen-glucose deprivation and reoxygenation (OGD/R).
Methods: Mouse brain microvascular endothelial cells (bEnd.3) were exposed to OGD/R to induce barrier dysfunction following treatment with S1PR5-specific agonist A971432 or lentivirus-mediated transfection with a S1PR5-specific siRNA, a S1PR5-overexpressing plasmid, or their respective negative control sequences. The changes in viability and endothelial barrier permeability of the treated cells were evaluated with CCK-8 assay and FITC-dextran permeability assay; the levels of intracellular reactive oxygen species (ROS) and localization and expression levels of the proteins related with barrier function and oxidative stress were detected using immunofluorescence staining, DCFH-DA probe and Western blotting.
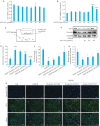
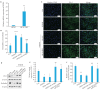
Results: S1PR5 activation obviously enhanced viability of bEnd.3 cells exposed to OGD/R (P<0.0001). Both activation and overexpression of S1PR5 reduced FITC-dextran leakage, while S1PR5 knockdown significantly increased FITC-dextran leakage in the exposed bEnd.3 cells. Activation and overexpression of S1PR5 both increased the cellular expressions of the barrier proteins ZO-1 and occludin, while S1PR5 knockdown produced the opposite effect. In cells exposed to OGD/R, ROS production was significantly reduced by S1PR5 activation and overexpression but increased following S1PR5 knockdown. Overexpression of S1PR5 obviously increased the expressions of the antioxidant proteins Nrf2, HO-1 and SOD2 in the exposed cells.
Conclusions: S1PR5 activation and overexpression significantly improve cell viability and reduce permeability of a mouse brain microvascular endothelial cell model of OGD/R, the mechanism of which may involve the reduction in ROS production and upregulation of the antioxidant proteins.
目的: 探讨鞘氨醇1-磷酸受体5(S1PR5)对氧糖剥夺及复氧复糖(OGD/R)诱导的小鼠脑微血管内皮细胞屏障功能的影响及相关机制。方法: 体外培养小鼠脑微血管内皮细胞bEnd.3,使用OGD/R诱导屏障功能障碍,分别使用S1PR5特异性激动剂A971432、敲低S1PR5的siRNA及过表达S1PR5的慢病毒进行干预。设置对照组:bEnd.3正常培养;OGD/R组:bEnd.3进行OGD/R;A971432组:OGD/R组+A971432;siNC组:转染siNC+OGD/R;siS1PR5组:转染siS1PR5+OGD/R;OE NC组:感染慢病毒LV5-NC+OGD/R;OE S1PR5组:感染慢病毒LV5-S1PR5+OGD/R。RT-qPCR法分别检测敲低或过表达S1PR5的效率;采用CCK-8检测在不同培养条件下bEnd.3细胞的活力;采用FITC-dextran渗透法检测内皮屏障渗透性;采用细胞免疫荧光法检测蛋白的定位及表达;DCFH-DA探针法检测细胞内活性氧水平;Western blotting法检测蛋白的表达水平。结果: CCK-8结果显示激动S1PR5可以增加OGD/R下调的细胞活力(P<0.0001),FITC-dextran渗透法结果显示激动和过表达S1PR5可减少FITC-dextran的渗漏(P<0.001),而敲低S1PR5增加FITC-dextran的渗漏(P<0.001)。Western blotting及免疫荧光结果显示,与OGD/R组相比,激动和过表达S1PR5可以增加屏障蛋白ZO-1(P<0.05)、Occludin(P<0.05)的表达,而敲低S1PR5会下调ZO-1和Occludin(P<0.05)的表达。ROS检测结果显示,激动和过表达S1PR5能减少ROS的产生,而敲低S1PR5会增加ROS产生。Western blotting检测发现过表达S1PR5可以增加抗氧化蛋白Nrf2(P<0.0001)、HO-1(P<0.0001)、SOD2(P<0.01)的表达。结论: S1PR5受体的激动剂干预及基因过表达可显著改善OGD/R模型诱导的活力减少及通透性增加,而基因敲低S1PR5则加剧OGD/R诱导的功能损伤,其机制可能与减少ROS,上调抗氧化蛋白表达相关。.
Keywords: blood-brain barrier dysfunction; oxidative stress; oxygen-glucose deprivation and reoxygenation; sphingosine 1-phosphate receptor 5.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
