[Pirfenidone inhibits bladder cancer xenograft growth in mice by regulating regulatory T cells]
- PMID: 40673314
- PMCID: PMC12268924
- DOI: 10.12122/j.issn.1673-4254.2025.07.18
[Pirfenidone inhibits bladder cancer xenograft growth in mice by regulating regulatory T cells]
Abstract
Objectives: To investigate the inhibitory effect of pirfenidone (PFD) on growth of bladder cancer xenograft and its regulatory effect on Treg cells in tumor-bearing mice.
Methods: Thirty-two C57BL/6 mice bearing ectopic bladder tumors were randomized into control and PFD groups (n=16). In PFD group, PFD was administered orally at the daily dose of 500 mg/kg, and tumor growth and survival of the mice were monitored. After treatment for 21 days, the tumors and vital organs were harvested for analysis. Immunohistochemistry was used to assess CD3, CD4, CD8, and FOXP3 expressions in the tumors. Flow cytometry and RT-qPCR were used to analyze the percentage of CD4⁺CD25⁺FOXP3⁺ Treg cells and IL-2, IL-10, and IL-35 expressions in the tumors and spleens; organ damage of the mice was examined with HE staining.
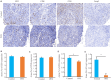
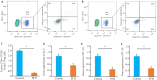
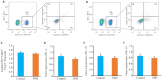
Results: Compared with the control group, the PFD-treated mice exhibited significantly lower tumor growth rate with smaller tumor volumes at day 21, along with improved survival at day 28. Immunohistochemistry revealed no significant differences in the infiltration of CD3⁺ and CD8⁺ cells between the two groups, but the percentages of CD4⁺ and FOXP3⁺ cells were significantly lower in the tumors of PFD-treated mice. Flow cytometric analysis confirmed a decrease in CD4⁺CD25⁺FOXP3⁺ Treg cells in the tumors from PFD-treated mice, which also had reduced expression levels of IL-2, IL-10 and IL-35 mRNAs in the tumors. No significant differences were found in Treg cell populations or cytokine expressions in the spleen tissues between the two groups. HE staining showed obvious organ damage in neither of the groups.
Conclusions: PFD inhibits bladder cancer growth and enhances survival of tumor-bearing mice possibly by suppressing Treg cells in the tumor microenvironment.
目的: 观察吡非尼酮(PFD)对小鼠异位膀胱肿瘤的抑制和肿瘤微环境调节性T细胞(Treg)的调控作用。方法: 构建C57BL/6小鼠异位膀胱肿瘤模型共32只,随机分为对照组和吡非尼酮(PFD)组,16只/组。对照组予正常饮食,PFD组小鼠建模后每天按500 mg/kg口服PFD。对比各组小鼠生存率和肿瘤生长速度,药物干预21 d后两组各随机留取6只小鼠的肿瘤、脾脏、肝脏、肺脏、心脏及肾脏等组织,免疫组化染色(IHC)检测肿瘤组织CD3、CD4、CD8和FOXP3等表达情况,流式细胞仪和PCR检测肿瘤和脾脏组织中CD4+CD25+FOXP3+ Treg细胞数量和IL-2、IL-10、IL-35等Treg细胞相关因子的表达。H&E染色观察小鼠脏器的损伤。结果: 与对照组相比,PFD组小鼠肿瘤相对生长速率及21d肿瘤体积减小(P<0.01),28 d生存率提高(P<0.05)。IHC结果显示两组小鼠肿瘤组织中CD3+和CD8+的细胞数量差别无统计学意义(P<0.05),但PFD组小鼠肿瘤组织中CD4+和Foxp3+的细胞数量均低于对照组(P<0.05)。流式细胞学分析证实PFD组小鼠肿瘤组织中CD4+CD25+Foxp3+细胞数和Treg细胞相关因子IL-2,IL-10和IL-35的基因表达水平均低于对照组(P<0.05),但两组小鼠脾脏组织Treg细胞数量及相关因子表达差异无统计学意义(P>0.05)。H&E染色结果显示两组小鼠脏器组织均无明显损伤。结论: PFD可显著抑制小鼠膀胱癌生长,提高生存率,其效应可能与肿瘤微环境Treg细胞的抑制相关。.
Keywords: bladder cancer; immune microenvironment; pirfenidone; regulatory T cells.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Xu J, Zhang H, Zhang L, et al. Real-world effectiveness and safety of RC48-ADC alone or in combination with PD-1 inhibitors for patients with locally advanced or metastatic urothelial carcinoma: a multicenter, retrospective clinical study[J]. Cancer Med, 2023, 12(23): 21159-71. doi: 10.1002/cam4.6680 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
