TCEP-Enabled Click Modification of Glycidyl-Bearing Polymers with Biorelevant Sulfhydryl Molecules: Toward Chemoselective Bioconjugation Strategies
- PMID: 40673360
- PMCID: PMC12344704
- DOI: 10.1021/acs.biomac.5c00766
TCEP-Enabled Click Modification of Glycidyl-Bearing Polymers with Biorelevant Sulfhydryl Molecules: Toward Chemoselective Bioconjugation Strategies
Abstract
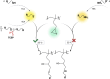
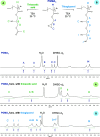
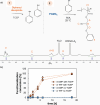
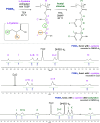
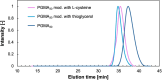
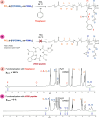
Thiol-epoxy ring opening is a highly efficient and versatile click reaction for postpolymerization modification, ideal for the conjugation of sulfhydryl-containing biomolecules. This study investigated the reactivity of thiols, disulfides, and amines toward glycidyl-bearing polymers, aiming to optimize thiol conjugation using tris(2-carboxyethyl)phosphine (TCEP) as a disulfide-reducing agent. Epoxide groups were introduced via glycidyl methacrylate (GMA) polymerized by ATRP to yield PGMA homopolymers and poly(ε-caprolactone) (PCL)-based block copolymers. 1H NMR confirmed quantitative thiol functionalization, while amines showed poor reactivity. l-cysteine conjugation further demonstrated the reaction's chemoselectivity. Thioglycerol conjugation yielded poly(2-hydroxy-3-(thioglycerol)propyl methacrylate) (PTGMA), a highly hydroxylated PEG alternative. Functionalization was extended to PCL-b-PGMA and PEGMA-based copolymers, forming amphiphilic nanoparticles via nanoprecipitation. Sequential modification with thioglycerol and the cRGD peptide yielded bioactive, size-controlled nanocarriers. Overall, a robust strategy has emerged for synthesizing multifunctional polymeric nanomaterials. Its compatibility with equimolar reactants under ambient conditions makes it particularly suited for the efficient incorporation of sensitive, high-value biomolecules into targeted drug delivery systems.
Figures


References
-
- Geng Z., Shin J. J., Xi Y., Hawker C. J.. Click Chemistry Strategies for the Accelerated Synthesis of Functional Macromolecules. J. Polym. Sci. 2021;59:963–1042. doi: 10.1002/pol.20210126. - DOI
-
- Muzammil E. M., Khan A., Stuparu M. C.. Post-Polymerization Modification Reactions of Poly(Glycidyl Methacrylate)s. RSC Adv. 2017;7:55874–55884. doi: 10.1039/C7RA11093F. - DOI
-
- Wang X., Yun W., Jiang W., Wang D., Zhang L., Tang J.. An Amphiphilic Non-Viral Gene Vector Prepared by a Combination of Enzymatic Atom Transfer Radical Polymerization and Enzymatic Ring-Opening Polymerization. RSC Adv. 2017;7(16):9926–9932. doi: 10.1039/C6RA28650J. - DOI
-
- Chen N., Zhang C., Liu Y., Dong X., Sun Y.. Cysteine-Modified Poly(Glycidyl Methacrylate) Grafted onto Silica Nanoparticles: New Supports for Significantly Enhanced Performance of Immobilized Lipase. Biochem. Eng. J. 2019;145:137–144. doi: 10.1016/j.bej.2019.02.018. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

