Structural and functional connectivity in tau mutation carriers: from presymptomatic to symptomatic frontotemporal dementia
- PMID: 40673371
- PMCID: PMC12268314
- DOI: 10.1002/alz.70367
Structural and functional connectivity in tau mutation carriers: from presymptomatic to symptomatic frontotemporal dementia
Abstract
Introduction: Microtubule-associated protein tau (MAPT) mutations cause frontotemporal dementia (FTD), characterised by behavioural, language, and motor impairments due to brain connectivity disruptions. We investigated structural and functional connectivity in 86 mutation carriers and 272 controls to map connectivity changes at different disease stages.
Methods: The CDR Dementia Staging Instrument plus National Alzheimer's Coordinating Center (NACC) Behaviour and Language domains (CDR plus NACC FTLD) stratified carriers into three groups: asymptomatic, prodromal, and symptomatic. We extracted measures of cortical thickness, white matter integrity, and functional connectivity, which were compared between each carrier group and controls using linear mixed models.
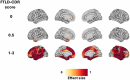
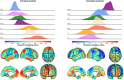
Results: Early isolated functional disruptions in salience/visual networks were present in asymptomatic carriers, along with anterior cingulate gray matter reductions. In prodromal carriers, functional changes extended to other networks, with additional structural damage in temporal poles/cingulate.
Discussion: This study shows that functional networks likely drive lifelong compensation for a genetically determined disease, manifesting clinically when structural damage reaches a critical threshold. This supports connectivity measures as potential biomarkers for MAPT-related neurodegeneration.
Highlights: Our findings reveal the progressive and staged nature of structural and functional connectivity alterations in MAPT mutation carriers, with distinct patterns at each disease stage. In asymptomatic carriers, we identified early functional connectivity alterations in salience and visual networks, despite preserved white matter and only subtle gray matter atrophy. These appear to represent both response to pathology and possible compensatory mechanisms. In prodromal carriers, functional connectivity alterations were accompanied by structural damage, including cortical atrophy and white matter tract disruptions, in regions directly connected to early-affected networks. The sequential progression, from functional connectivity changes to structural degeneration, aligns with the hypothesis that tau propagates along axonal connections, disrupting neural network integrity before measurable atrophy occurs. We propose a theoretical data-driven model of biomarker evolution in MAPT mutation carriers, highlighting functional disruptions as early indicators and structural damage as a later-stage hallmark. These connectivity biomarkers have the potential to inform therapeutic strategies and clinical trial design.
Keywords: MAPT; functional connectivity; genetic frontotemporal dementia; graph analysis; gray matter; macroscale organization; mutation; neurodegeneration; tau; tau pathology; white matter.
© 2025 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
The authors declare no conflicts of interest. Author disclosures are available in the Supporting Information.
Figures




References
MeSH terms
Substances
Grants and funding
- 09-02-03-00/Dioraphte Foundation
- 2009/Association for Frontotemporal Dementias Research
- HCMI 056-13-018/Netherlands Organization for Scientific Research
- 733 051 042/Deltaplan Dementie
- 10510032120002/ZonMw Onderzoeksprogramma Dementie
- Alzheimer Nederland
- ARUK-CRF2017B-2/Alzheimer's Research UK Clinical Research
- 20143810/Fundació Marató de TV3
- 529-2014-7504/Neurodegenerative Disease Research-Prefrontals Vetenskapsrådet Dnr
- Neurodegenerative Disease Research-GENFI-PROX
- 2018-02754/Vetenskapsrådet
- FTD Inititative-Schörling Foundation
- Alzheimer Foundation
- Brain Foundation
- Dementia Foundation
- 733051042/Neurodegenerative Disease Research and the Italian Ministry of Health (PreFrontALS)
- Mady Browaeys
- Research into Frontotemporal Dementia
- Deutsche Forschungsgemeinschaft German Research Foundation
- EXC 2145 SyNergy-ID 390857198/Germany's Excellence Strategy within the framework of the Munich Cluster for Systems Neurology
- Germany's Federal Ministry of Education and Research (BMBF)
- 327387/Canadian Institute of Health Research
- Canadian Institute of Health Research operating
- Weston Brain Institute and Ontario Brain Institute
- 103838/WT_/Wellcome Trust/United Kingdom
- 220258/WT_/Wellcome Trust/United Kingdom
- Bluefield Project
- Cambridge University Centre for Frontotemporal Dementia
- MC_UU_00030/14/MRC_/Medical Research Council/United Kingdom
- MR/T033371/1/MRC_/Medical Research Council/United Kingdom
- NIHR203312/National Institute for Health Research Cambridge Biomedical Research Centre
- Tau Consortium
- PI19/01637/Carlos III Health Institute
- University College London Hospitals Biomedical Research Centre
- MR/M008525/1/MRC Clinician Scientist Fellowship
- Miriam Marks Brain Research UK
- France Alzheimer
- Fondation Recherche Alzheimer
- Fondation Philippe Chatrier
- No. 739510/European Reference Network for Rare Neurological Diseases (ERN-RND)
- 2019-02248/Neurodegenerative Disease Research GENFI-PROX
- Association pour la Recherche sur la Sclérose Latérale Amyotrophique et autres Maladies du Motoneurone
- Fondation Vaincre Alzheimer
- National Institute for Health and Care Research
- Rosita Gomez association
- Region Stockholm ALF-project

