Prognostic Value of Deep Learning-Extracted Tumor-Infiltrating Lymphocytes in Esophageal Cancer: A Multicenter Retrospective Cohort Study
- PMID: 40673386
- PMCID: PMC12268311
- DOI: 10.1002/cam4.71054
Prognostic Value of Deep Learning-Extracted Tumor-Infiltrating Lymphocytes in Esophageal Cancer: A Multicenter Retrospective Cohort Study
Abstract
Background: Tumor-infiltrating lymphocytes (TILs) have been proven to be important prognostic factors for various tumors. However, their prognostic significance within the context of esophageal squamous cell carcinoma (ESCC) remains inadequately explored. This study aims to assess the prognostic potential of TILs in ESCC using deep learning (DL) methods.
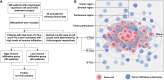
Materials and methods: We retrospectively enrolled 626 pathologically confirmed ESCC patients from two research centers. Their digital whole-slide imaging (WSI) and corresponding clinical information were collected. Subsequently, the DL method was employed to identify the tumor margin and TILs within the WSI. Tissue was divided into intratumor, peritumoral, and stromal regions based on their distance from the tumor margin. TILs were counted in each region. Optimal cut-off values of TILs were determined using the X-tile software. To mitigate selection bias and intergroup heterogeneity, a propensity score matching (PSM) analysis was employed. Survival analysis was performed using Kaplan-Meier curves and the log-rank test. The Cox proportional hazards regression model was used to identify independent prognostic factors.
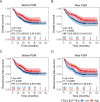
Results: We classified patients based on the cell counts and cut-off values of intratumor-infiltrating lymphocytes (I-TILs) and peritumoral infiltrating lymphocytes (P-TILs). Patients with high I-TILs and P-TILs were defined as those whose counts of both I-TILs and P-TILs exceeded the determined cutoff value. Patients with high I-TILs and P-TILs showed significantly better overall survival (OS, p = 0.0092) and recurrence-free survival (RFS, p = 0.0088) than patients with low I-TILs and P-TILs after PSM. Multivariable Cox proportional hazards regression further supported this conclusion and recognized I-TILs and P-TILs as independent prognostic factors (p = 0.0136, hazard ratio = 0.63 for OS; p = 0.0098, hazard ratio = 0.63 for RFS).
Conclusion: In the present study, we identified the quantitative distribution of TILs in ESCC patients with the help of the DL method. We established that I-TILs and P-TILs serve as independent prognostic factors for these patients. Further studies should focus on the lymphocyte subgroups and make better use of the spatial information to improve the predictive efficacy of TILs.
Keywords: deep learning; esophageal squamous cell carcinoma; prognostic factors; tumor‐infiltrating lymphocytes.
© 2025 The Author(s). Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- NCCN Guidelines for Patients: Esophageal Cancer , “Esophageal Cancer” (2022), https://www.nccn.org/patients/guidelines/content/PDF/esophageal‐patient.pdf.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

