Supramolecular chemistry for optical detection and delivery applications in living plants
- PMID: 40673397
- PMCID: PMC12268990
- DOI: 10.1039/d4cs00500g
Supramolecular chemistry for optical detection and delivery applications in living plants
Abstract
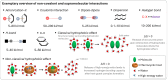
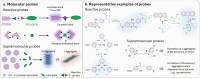
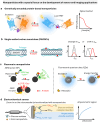
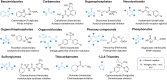
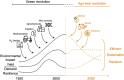
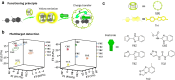
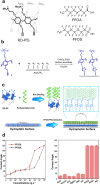
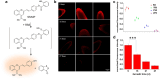
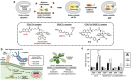
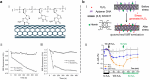
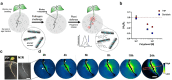
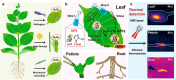
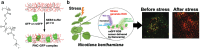
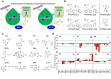
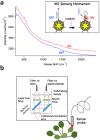
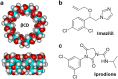
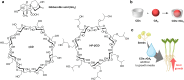
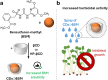
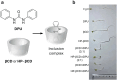
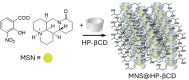
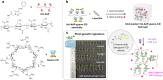
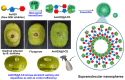
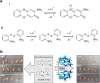
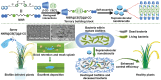
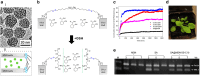
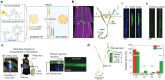
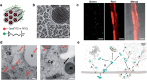
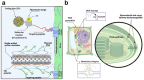
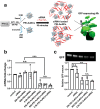
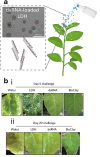
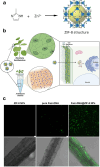
Over the past century, modern agriculture, through the use of synthetic fertilisers, pesticides, and improved plant breeding, has greatly increased food production. However, this progress has brought serious environmental consequences, including excessive water use and harmful pesticide exposure. In addition, future farming must adapt to the growing challenges posed by climate change and natural disasters through more sustainable practices and resilient crop management. In this context, emerging supramolecular strategies offer promising alternatives through responsive molecular assemblies capable of precise sensing and controlled delivery. In this review, we thus discuss the application of supramolecular chemistry principles to plant science and agriculture, with a particular emphasis on the design and implementation of host-guest systems, chemosensors, and supramolecular (nano)delivery vehicles for use in living plants. We report and analyse recent advances in sensing and monitoring of plant processes, the detection of pesticides, the preparation of safer and more effective supramolecular pesticides, and nucleic acid-based crop protection strategies, highlighting key design principles specific to the plant biological context. Moreover, key challenges are discussed regarding the application of supramolecular systems to plants, and examples are highlighted to promote new interdisciplinary strategies for designing next-generation tools for real-time, in vivo plant studies and sustainable crop management.
Conflict of interest statement
There are no conflicts to declare.
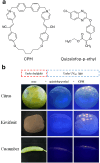
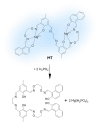
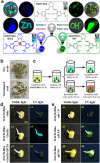
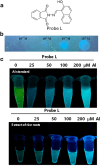
Figures











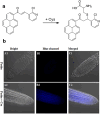

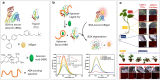
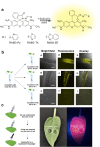
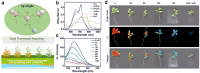





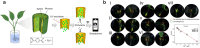



















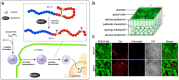
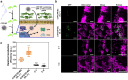
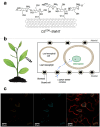
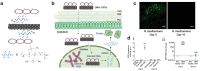






References
-
- Erisman J. W. Sutton M. A. Galloway J. Klimont Z. Winiwarter W. Nat. Geosci. 2008;1:636–639.
-
- Whalen J. M. Matlin S. A. Holme T. A. Stewart J. J. Mahaffy P. G. ACS Sustainable Chem. Eng. 2022;10:12933–12947.
-
- Sassenrath G. F. Heilman P. Luschei E. Bennett G. L. Fitzgerald G. Klesius P. Tracy W. Williford J. R. Zimba P. V. Renewable Agric. Food Syst. 2008;23:285–295.
-
- Shan-e-Ali Zaidi S. Vanderschuren H. Qaim M. Mahfouz M. M. Kohli A. Mansoor S. Tester M. Science. 2019;363:1390–1391. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

