Retinal Mid-Peripheral Capillary Free Zones Are Enlarged in Diabetic Patients With No Evidence of Clinical Retinopathy
- PMID: 40673733
- PMCID: PMC12282639
- DOI: 10.1167/tvst.14.7.10
Retinal Mid-Peripheral Capillary Free Zones Are Enlarged in Diabetic Patients With No Evidence of Clinical Retinopathy
Abstract
Purpose: To compare the width of the mid-peripheral capillary free zones (CFZs; periarteriole and perivenule) between diabetics with no diabetic retinopathy (DR) versus controls.
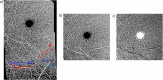
Methods: The 20° × 20° optical coherence tomography angiography images of paired arterioles, venules, and their adjacent capillaries within the macular and inferomacular regions of the superficial vascular plexus were obtained from 46 eyes of 28 diabetics with no DR (mean age, 59 years; range, 40-71 years) and 46 eyes of 31 controls (mean age, 59 years; range, 46-78 years). There was no significant difference in age between groups (P = 0.77). The macular and inferomacular images were montaged to generate a wider field of view, followed by the application of a vesselness filter and Otsu thresholding. The mid-peripheral CFZ width was calculated using previously established MATLAB algorithms. Generalized linear mixed models were used to compare the mid-peripheral CFZs between groups, accounting for correlation between eyes.
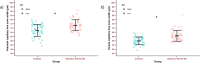
Results: The periarteriole CFZ width was greater in diabetics with no DR (73.3 ± 6.49 µm) compared to controls (67.3 ± 7.08 µm; P < 0.001, Cohen's d = 0.88). Similarly, the perivenule CFZ width was larger in diabetics with no DR (60.8 ± 6.40 µm) compared to controls (54.8 ± 4.58 µm; P < 0.001, Cohens' d = 1.08).
Conclusions: Our results demonstrate larger mid-peripheral CFZ width in diabetics with no DR. The mid-peripheral CFZs show promise as a potential novel retinal vascular biomarker for early DR detection.
Translational relevance: Our study shows the potential clinical utility of the mid-peripheral CFZs for early DR detection.
Conflict of interest statement
Disclosure:
Figures





References
-
- Elsner AE, King BJ.. Screening for macular disorders: the optometrist's perspective. Clin Optom. 2015; 7: 15–38.
-
- Early Treatment Diabetic Retinopathy Study Research Group. Grading diabetic retinopathy from stereoscopic color fundus photographs—an extension of the modified Airlie House classification. ETDRS Report Number 10. Ophthalmology. 1991; 98(5): 786–806. - PubMed
-
- Wilkinson CP, Ferris FL III, Klein RE, et al.. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003; 110(9): 1677–1682. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

