Preventing Secondary Lymphedema: A Systematic Review and Meta-Analysis on the Efficacy of Immediate Lymphovenous Anastomosis
- PMID: 40673745
- PMCID: PMC12455550
- DOI: 10.1002/jso.70046
Preventing Secondary Lymphedema: A Systematic Review and Meta-Analysis on the Efficacy of Immediate Lymphovenous Anastomosis
Abstract
Background: Secondary lymphedema is a debilitating condition following oncologic lymphadenectomy. Despite advancements in rehabilitation and microsurgical interventions, there is no cure for lymphedema. Performing a lymphovenous anastomosis (LVA) at the time of a regional node dissection has been purported to reduce the risks of secondary lymphedema; however, there are conflicting studies and no clear consensus about the routine use of LVA for preventing lymphedema after lymphadenectomy. The present study aims to perform a comprehensive review and meta-analysis on immediate LVA for the prevention of secondary lymphedema.
Methods: A systematic review and literature search were performed using PubMed, Embase, Web of Science, and Cochrane databases. Studies evaluating primary or immediate LVA in oncologic surgery were included. Studies with a control group were included in the meta-analysis.
Results: Overall, 39 studies, including 3697 patients (1,722 LVA; 1975 control), met inclusion criteria. Seventeen of the studies were included in the meta-analysis. Pooled analysis across all studies revealed a secondary lymphedema incidence of 7.1% in the LVA cohort versus 35.0% in controls. Meta-analysis demonstrated a significant reduction in lymphedema risk with immediate LVA (RR: 0.31). Subgroup analysis confirmed strong protective effects in breast cancer patients (RR: 0.28) and a significant but lesser benefit in dermatologic malignancies (RR: 0.35).
Conclusion: Based on the current literature, immediate LVA at time of lymphadenectomy significantly reduces the risk of secondary lymphedema in patients undergoing oncologic treatment. Given these findings, patients undergoing multimodal oncologic treatment including radiation and surgical lymphadenectomy should be considered candidates for immediate LVA.
Keywords: immediate lymphovenous anastomosis; lymph node surgery; lymphatic bypass; lymphedema; microsurgery; oncologic reconstruction; supermicrosurgery.
© 2025 The Author(s). Journal of Surgical Oncology published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



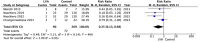
References
-
- Friedman J. F., Sunkara B., Jehnsen J. S., Durham A., Johnson T., and Cohen M. S., “Risk Factors Associated With Lymphedema After Lymph Node Dissection in Melanoma Patients,” American Journal of Surgery 210 (2015): 1178–1184. - PubMed
-
- Shah C., Wilkinson J. B., Baschnagel A., et al., “Factors Associated With the Development of Breast Cancer–Related Lymphedema After Whole‐Breast Irradiation,” International Journal of Radiation Oncology, Biology, Physics 83 (2012): 1095–1100. - PubMed
-
- Cormier J. N., Askew R. L., Mungovan K. S., Xing Y., Ross M. I., and Armer J. M., “Lymphedema Beyond Breast Cancer,” Cancer 116 (2010): 5138–5149. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

