Extracellular Vesicles Derived From Streptococcus anginosus Aggravate Lupus Nephritis by Triggering TLR2-MyD88-NF-κB Signalling in NK Cells
- PMID: 40673809
- PMCID: PMC12269530
- DOI: 10.1002/jev2.70134
Extracellular Vesicles Derived From Streptococcus anginosus Aggravate Lupus Nephritis by Triggering TLR2-MyD88-NF-κB Signalling in NK Cells
Abstract
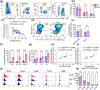
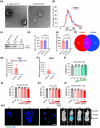
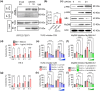
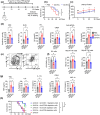
Systemic lupus erythematosus (SLE) has been linked to gut microbiome dysbiosis, notably an overabundance of Streptococcus anginosus; however, the impact of this microbial imbalance on disease pathogenesis remains unclear. Here, we investigated the contribution of S. anginosus-derived extracellular vesicles (SA-EVs) to SLE progression, with an emphasis on lupus nephritis (LN). Fifty-four SLE patients and 43 healthy controls (HC) were recruited. The faecal, blood and serum samples from participants were collected. SLE disease activity (SLEDA) was evaluated by the SLEDA Index (SLEDAI). Stool S. anginosus abundance was quantified by quantitative PCR, NK cell activation by flow cytometry and serum proinflammatory cytokines profile by ELISA. Lupus-prone MRL/lpr mice were orally administered SA-EVs to evaluate in vivo inflammatory responses, renal NK cell activation and renal histopathological changes. S. anginosus levels were significantly elevated in SLE patients relative to HC, positively correlated with SLEDAI scores and NK cell cytotoxicity. In vitro, SA-EVs stimulation of patient NK cells significantly heightened proinflammatory mediator production (granzyme B, TNF-α), increased cytotoxicity and downregulated inhibitory receptors (TIM-3, NKG2A, TIGIT) compared to control EVs from S. Salivarius (SS-EVs). Mechanistically, lipoteichoic acid (LTA) within SA-EVs engaged Toll-like receptor 2 (TLR2) on NK cells, activating MyD88/NF-κB signalling pathway. In MRL/lpr mice, SA-EVs treatment increased renal immune complex deposition, upregulated renal NK cell activation markers (NKp44, NKp46), and exacerbated LN pathology with greater immune cell infiltration and inflammatory cytokine levels. Furthermore, NK cell depletion with anti-NK1.1 antibodies significantly prolonged survival in SA-EVs administered mice. Thus, SA-EVs exacerbate SLE by hyperactivating NK cells via the TLR2-MyD88-NF-κB pathway, leading to amplified systemic inflammation and aggravated LN. These findings underscore the potential of targeting SA-EVs for therapeutic intervention in SLE.
Keywords: TLR2‐ MyD88‐NF‐κB signalling; bacterial extracellular vesicle; lupus nephritis (LN); natural killer cells (NK); systemic lupus erythematosus (SLE).
© 2025 The Author(s). Journal of Extracellular Vesicles published by Wiley Periodicals, LLC on behalf of the International Society for Extracellular Vesicles.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Mesenchymal stem cells-derived extracellular vesicles ameliorate lupus nephritis by regulating T and B cell responses.Stem Cell Res Ther. 2024 Jul 18;15(1):216. doi: 10.1186/s13287-024-03834-w. Stem Cell Res Ther. 2024. PMID: 39020448 Free PMC article.
-
Expression pattern and clinical significance of MerTK on circulating NK cells in systemic lupus erythematosus.Lupus Sci Med. 2025 Jun 19;12(1):e001407. doi: 10.1136/lupus-2024-001407. Lupus Sci Med. 2025. PMID: 40541265 Free PMC article.
-
Mahonia bealei (Fort.) Carr. Leaf extract modulates the TLR2/MyD88/NF-κB signaling pathway to inhibit PGN-induced inflammation in RAW264.7 cells.J Ethnopharmacol. 2025 Mar 26;344:119510. doi: 10.1016/j.jep.2025.119510. Epub 2025 Feb 17. J Ethnopharmacol. 2025. PMID: 39971016
-
Are anti-nucleosome antibodies a better diagnostic marker than anti-dsDNA antibodies for systemic lupus erythematosus? A systematic review and a study of metanalysis.Autoimmun Rev. 2012 Dec;12(2):97-106. doi: 10.1016/j.autrev.2012.07.002. Epub 2012 Jul 15. Autoimmun Rev. 2012. PMID: 22810055
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
References
MeSH terms
Substances
Grants and funding
- Subproject of National Key R&D Project of Ministry of Science and Technology
- President's Fund of Nanfang Hospital, Southern Medical University
- Guangzhou Basic and Applied Basic Research Foundation
- Guangdong Basic and Applied Basic Research Foundation
- 82025024/National Science Fund for Distinguished Young Scholars
LinkOut - more resources
Full Text Sources
Research Materials

