Roadblocks of Urinary EV Biomarkers: Moving Toward the Clinic
- PMID: 40673839
- PMCID: PMC12269535
- DOI: 10.1002/jev2.70120
Roadblocks of Urinary EV Biomarkers: Moving Toward the Clinic
Abstract
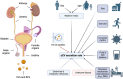
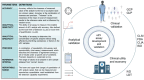
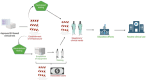
Despite remarkable interest in the biomarker potential of urinary extracellular vesicles (uEVs) and the identification of numerous promising candidates, their clinical translation still presents multiple challenges. The opportunities for successful translation are obvious, yet the main roadblocks on the way have hardly been systematically considered and more coordinated approaches are needed to overcome them. In the present review article, we have identified the most relevant roadblocks of clinical translation of urinary EV-based biomarkers and discuss possible solutions to overcome them. These roadblocks are categorized as fundamental and technical but also related to development of novel biomarker assays and clinical acceptance. In addition, hurdles within the regulatory approval process are discussed. It is clear that various roadblocks to clinical translation of urinary EV biomarkers exist; however, they are addressable by promoting rigor and reproducibility as well as collaboration between basic and clinical scientists, clinicians, industry and regulatory bodies. Moreover, knowledge of obstacles for assay development and regulatory requirements should already be considered when developing a new biomarker to maximize the chance of successful translation. This review presents not only a status quo, but also a roadmap for the further development of the field.
Keywords: biomarkers; bladder; exosomes; extracellular vesicles; kidney; liquid biopsy; prostate; translation; urine.
© 2025 The Author(s). Journal of Extracellular Vesicles published by Wiley Periodicals LLC on behalf of International Society for Extracellular Vesicles.
Conflict of interest statement
Desmond Pink is the founder and employee at Nanostics Inc. Fabrice Lucien discloses research funding and financial compensation from NaNotics LLC, licensing agreement and royalties from Early Is Good and consulting for Mursla Bio. Marvin Droste discloses speaking fees from Novartis, outside of the published work. All other authors report no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
- Fundación Mutua Madrileña and Fundación SENEFRO
- 2021-03424/Canadian Network for Research and Innovation in Machining Technology, Natural Sciences and Engineering Research Council of Canada
- 722ICTSPM19/American Diabetes Association
- Queensland Health Clinical Research Fellowship and Metro North Clinician Research Fellowship
- Intramural Program NIH
- R01 CA234557/CA/NCI NIH HHS/United States
- R01CA234557/RC/CCR NIH HHS/United States
- 920575/Kidney Foundation of Canada
- R01 CA287075/CA/NCI NIH HHS/United States
- RD21/0005/0001/European Regional Development Fund
- PI20/01103/European Regional Development Fund
- 101125504/H2020 European Research Council
- PIPF-2022/SAL-GL-25760/Comunidad de Madrid
- 186237/CIHR/Canada
- J3-50117/The Slovenian Research and Innovation Agency
- R01CA287075/RC/CCR NIH HHS/United States
- PI20/01103/Instituto de Salud Carlos III
- RD21/0005/0001/Instituto de Salud Carlos III
LinkOut - more resources
Full Text Sources

