A second-generation molecular clamp stabilised bivalent candidate vaccine for protection against diseases caused by respiratory syncytial virus and human metapneumovirus
- PMID: 40674411
- PMCID: PMC12270161
- DOI: 10.1371/journal.ppat.1013312
A second-generation molecular clamp stabilised bivalent candidate vaccine for protection against diseases caused by respiratory syncytial virus and human metapneumovirus
Abstract
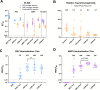
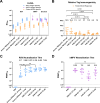
Respiratory syncytial virus (RSV) and human metapneumovirus (hMPV) are two medically important causes of respiratory tract infections and diseases. After more than five decades of research and development, vaccines have recently been approved for the prevention of lower respiratory tract disease caused by RSV. However, vaccines for hMPV remain in early-stage development. Here we describe the design and characterisation as well as pre-clinical development of a bivalent vaccine, VXB-241, comprised of the recombinantly expressed viral fusion proteins from both RSV and hMPV, stabilised in their pre-fusion conformation by combining the use of two technologies, the second-generation molecular clamp (MC2S) and key pre-fusion stabilizing mutations. Each of the two antigens were produced at high yield in a mammalian expression system and purified by an affinity capture resin specific to MC2S. Each antigen was demonstrated to adopt the pre-fusion conformation, which was stable for at least twelve months in liquid formulation at 2-8°C. Head-to-head evaluation in mouse immunogenicity studies showed that the VXB-241 candidate vaccine induced a neutralising immune response that was superior or equivalent to the pre-fusion stabilised comparator antigens for either RSV or hMPV, including the RSVPreF3 antigen of the licensed RSV vaccine, Arexvy (GSK). The results presented here have supported progression of VXB-241 into a Phase 1 clinical trial which commenced enrolment in August 2024 (ClinicalTrials.gov ID NCT06556147).
Copyright: © 2025 Young et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: Keith Joseph Chappell, Daniel Watterson and Paul Robert Young own intellectual property related to molecular clamp and shares in Vicebio. Keith Joseph Chappell, Julie Louise Dutton, Juana Magdalena, Frank Vandendriessche and Jean Smal are paid consultants for, and have shares/stock options in, Vicebio. Emmanuel Jules Hanon has shares/stock options and is the chief executive officer of Vicebio All other authors declare no competing interests.
Figures

References
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

