Inactivation of PI3K-C2α deregulates cell death pathways and sensitizes to endotoxic shock
- PMID: 40674428
- PMCID: PMC12304892
- DOI: 10.1073/pnas.2423358122
Inactivation of PI3K-C2α deregulates cell death pathways and sensitizes to endotoxic shock
Abstract
The organismal roles of the class II PI3K isoform PI3K-C2α remain poorly understood. Recent studies have found PI3K-C2α to promote arterial thrombosis and breast cancer metastasis, generating interest in this kinase as a drug target, with small molecule PI3K-C2α inhibitors now available. However, the consequences of systemic PI3K-C2α inactivation in the nondiseased, postnatal state are largely unknown. Here, we show that induction of genetic PI3K-C2α inactivation in adult mice is well tolerated, without adverse effects on normal physiology. Surprisingly, however, mice with inactive PI3K-C2α display strong sensitization to challenge with bacterial lipopolysaccharide (LPS), a model of endotoxic shock. This sensitization is recapitulated by vascular endothelial-specific deletion of PI3K-C2α. Furthermore, sensitization to LPS can be fully rescued by disabling extrinsic induction of cell death by combined caspase-8- and RIPK3 deficiency. These observations validate the tolerability of systemic PI3K-C2α inhibition in principle but reveal an unexpected role for PI3K-C2α in the regulation of extrinsic cell death pathways.
Keywords: PI3K; endotoxic shock; phosphoinositide; regulated cell death; vascular endothelia.
Conflict of interest statement
Competing interests statement:B.V. is a consultant for iOnctura (Geneva, Switzerland)., and shareholder of Open Orphan and Poolbeg Pharma (Dublin, Ireland).
Figures
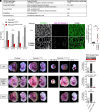
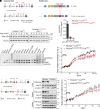
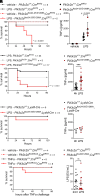
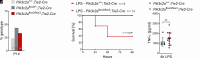

References
-
- Bilanges B., Posor Y., Vanhaesebroeck B., PI3K isoforms in cell signalling and vesicle trafficking. Nat. Rev. Mol. Cell Biol. 20, 515–534 (2019). - PubMed
-
- Selvadurai M. V., et al. , Disrupting the platelet internal membrane via PI3KC2alpha inhibition impairs thrombosis independently of canonical platelet activation. Sci. Transl. Med. 12, eaar8430 (2020). - PubMed
-
- Setiabakti N. M., Tarlac V., Larsson P., Hamilton J. R., PI3KC2alpha inhibition is antithrombotic in blood from hypercholesterolemic mice. J. Thromb. Haemost. 22, 249–254 (2024). - PubMed
-
- Mountford J. K., et al. , The class II PI 3-kinase, PI3KC2alpha, links platelet internal membrane structure to shear-dependent adhesive function. Nat. Commun. 6, 6535 (2015). - PubMed
MeSH terms
Substances
Grants and funding
- A27323/Cancer Research UK (CRUK)
- BB/R017972/1/UKRI | Biotechnology and Biological Sciences Research Council (BBSRC)
- 656778/EC | Horizon Europe | Excellent Science | HORIZON EUROPE Marie Sklodowska-Curie Actions (MSCA)
- C416/A29287/Cancer Research UK (CRUK)
- BB/W007460/1/UKRI | Biotechnology and Biological Sciences Research Council (BBSRC)
- GA:838559/EC | Horizon Europe | Excellent Science | HORIZON EUROPE Marie Sklodowska-Curie Actions (MSCA)
- BB/I007806/1/UKRI | Biotechnology and Biological Sciences Research Council (BBSRC)
- 294880/EC | European Research Council (ERC)
- MR/ S00811X/1/UKRI | Medical Research Council (MRC)
- 214342/Z/18/Z/Wellcome Trust (WT)
- SFB1399 [413326622] project A09/Deutsche Forschungsgemeinschaft (DFG)
- C23338/A25722/Cancer Research UK (CRUK)
- SFB1403 [414786233] project A10/Deutsche Forschungsgemeinschaft (DFG)
- SFB1403 [414786233] project A12/Deutsche Forschungsgemeinschaft (DFG)
- SFB1530 [455784452] project A03/Deutsche Forschungsgemeinschaft (DFG)
- 214342/WT_/Wellcome Trust/United Kingdom
- C23338/A15965/Cancer Research UK (CRUK)
- ALTF 1227-2014/European Molecular Biology Organization (EMBO)
- SFB1399 [413326622] project C06/Deutsche Forschungsgemeinschaft (DFG)
- SFB1530 [455784452] project B02/Deutsche Forschungsgemeinschaft (DFG)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

