Optimal dosing and duration of linezolid for the treatment of multidrug-resistant and rifampicin-resistant tuberculosis: an individual patient data meta-analysis
- PMID: 40675768
- PMCID: PMC12371315
- DOI: 10.1183/13993003.00315-2025
Optimal dosing and duration of linezolid for the treatment of multidrug-resistant and rifampicin-resistant tuberculosis: an individual patient data meta-analysis
Abstract
Background: The optimal dosing strategy of linezolid for treating multidrug-resistant and rifampicin-resistant tuberculosis remains unclear. We conducted an individual patient data meta-analysis to determine the optimal linezolid dosing strategy.
Methods: We searched for randomised controlled trials and prospective cohort studies on short-course all‑oral regimens containing linezolid for treating multidrug-resistant and rifampicin-resistant tuberculosis in PubMed, Embase and Scopus up to 31 August 2023. Patients were grouped according to linezolid dosing patterns. Time to treatment success and adverse events of grade 3 and higher were analysed using the Fine-Gray sub-distribution hazard model.
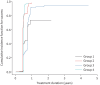
Results: Of 12 eligible studies, eight (four randomised controlled trials, four prospective studies) were included. Overall, 945 patients were grouped as follows: group 1 (600 mg·day-1 linezolid for 8 weeks), group 2 (600 mg·day-1 for 16 weeks, then 300 mg·day-1 for 8 weeks), group 3 (600 mg·day-1 for 39 weeks) and group 4 (1200 mg·day-1 for 25 weeks). Proportions of patients achieving treatment success were 59.1%, 90.4%, 91.3% and 96.0%, respectively. Compared with group 2, group 1 (adjusted sub-distribution hazard ratio (SHR) 0.24, 95% CI 0.08-0.71) and group 3 (adjusted SHR 0.36, 95% CI 0.16-0.81) had lower success rates. While group 4 showed no significant difference in treatment success versus group 2 (adjusted SHR 0.57, 95% CI 0.23-1.43), it had a higher rate of adverse events of grade 3 and higher (adjusted SHR 2.29, 95% CI 1.37-3.83).
Conclusion: A dosing strategy of 600 mg·day-1 linezolid for 16 weeks then 300 mg·day-1 for 8 weeks could be optimal for treating multidrug-resistant and rifampicin-resistant tuberculosis when considering effectiveness and safety.
Copyright ©The authors 2025.
Conflict of interest statement
Conflict of interest: M. Beumont and S. Foraida are employees of TB Alliance. R.A. Murphy reports payment for expert testimony and support for attending meetings from the University of Nevada. J-J. Yim has participated or is currently participating as the overall or institutional principal investigator in multiple clinical trials sponsored by Insmed Incorporated, AN2 Therapeutics and LigaChem Biosciences Inc. The remaining authors have no potential conflicts of interest to disclose.
Figures
Comment in
-
Linezolid for the treatment of drug-resistant tuberculosis.Eur Respir J. 2025 Aug 22;66(2):2500927. doi: 10.1183/13993003.00927-2025. Print 2025 Aug. Eur Respir J. 2025. PMID: 40846488 No abstract available.
References
-
- World Health Organization . Global Tuberculosis Report 2024. Geneva, World Health Organization, 2024.
-
- Nyang'wa B-T, Berry C, Kazounis E, et al. Short oral regimens for pulmonary rifampicin-resistant tuberculosis (TB-PRACTECAL): an open-label, randomised, controlled, phase 2B-3, multi-arm, multicentre, non-inferiority trial. Lancet Respir Med 2024; 12: 117–128. doi: 10.1016/S2213-2600(23)00389-2 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources