Purification and characterization of an antimicrobial compound against drug-resistant MRSA and VRE produced by Streptomyces levis strain HFM-2
- PMID: 40676119
- PMCID: PMC12271353
- DOI: 10.1038/s41598-025-10572-3
Purification and characterization of an antimicrobial compound against drug-resistant MRSA and VRE produced by Streptomyces levis strain HFM-2
Abstract
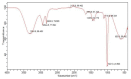
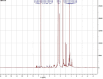
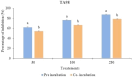
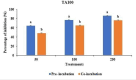
Due to high resistance to medicines, multidrug-resistant (MDR) bacterial pathogens, particularly MRSA (methicillin-resistant Staphylococcus aureus) and VRE (vancomycin-resistant enterococci), are a significant public health concern for treating nosocomial infections. Researchers are developing novel compounds responding to the global rise in MDR infections. This study aimed to extract, purify, and characterize bioactive metabolites from Streptomyces levis strain HFM-2, a human gut isolate, exhibiting strong antimicrobial activity against several MDR pathogenic bacteria and fungal phytopathogens. Ethyl acetate extract of S. levis strain HFM-2 was purified using silica-gel column chromatography and reverse-phase high-performance liquid chromatography. Structure elucidation of the purified antimicrobial compound was done by performing detailed analyses including MS, IR, and NMR. The bacteriostatic activity of the compound revealed interesting values against broad-spectrum MDR pathogens. The bacterial cell destruction was recorded through SEM and fluorescence microscopy analyses. HFM-2P is displayed to be non-mutagenic and non-cytotoxic to the normal cell line. However, dose-dependent cytotoxicity was observed against the HeLa cancer cell line and exhibited antimutagenic activity against Salmonella Typhimurium strains (TA98 and TA100). This study is the first to report antiproliferative, DNA protective potential, antimutagenic properties, and antimicrobial activity of a 2,6-disubstituted chromone derivative isolated from S. levis strain HFM-2 against drug-resistant MRSA, VRE, and fungal phytopathogens. Therefore, this essential compound could be a candidate for future research in the pharmaceutical and agricultural sectors.
Keywords: Streptomyces; Antimicrobial; Antimutagenicity; Antiproliferative; DNA nicking; Drug-resistant.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures





Similar articles
-
Therapeutic Potential of Salvianolic Acid B Produced by Streptomyces sp. M4 Against Multi-drug-Resistant Human Pathogens.Curr Microbiol. 2025 Jul 18;82(9):392. doi: 10.1007/s00284-025-04374-z. Curr Microbiol. 2025. PMID: 40679637
-
Purification and characterization of actinomycins from Streptomyces strain M7 active against methicillin resistant Staphylococcus aureus and vancomycin resistant Enterococcus.BMC Microbiol. 2019 Feb 19;19(1):44. doi: 10.1186/s12866-019-1405-y. BMC Microbiol. 2019. PMID: 30782119 Free PMC article.
-
Discovery and characterization of bioactive compounds from Limnophila aromatica: nevadensin and related flavonoids as potent antimicrobial agents.World J Microbiol Biotechnol. 2025 Aug 7;41(8):301. doi: 10.1007/s11274-025-04523-3. World J Microbiol Biotechnol. 2025. PMID: 40770451
-
A systematic review on natural products with antimicrobial potential against WHO's priority pathogens.Eur J Med Res. 2025 Jul 1;30(1):525. doi: 10.1186/s40001-025-02717-x. Eur J Med Res. 2025. PMID: 40597250 Free PMC article.
-
Interventions for the eradication of meticillin-resistant Staphylococcus aureus (MRSA) in people with cystic fibrosis.Cochrane Database Syst Rev. 2022 Dec 13;12(12):CD009650. doi: 10.1002/14651858.CD009650.pub5. Cochrane Database Syst Rev. 2022. PMID: 36511181 Free PMC article.
References
-
- Ganesan, G., Velayudhan, S. S. & David, J. S. R. Statistical optimization of medium constituents and conditions for improved antimicrobial compound production by marine Streptomyces sp. JRG-04. Arch. Biol. Sci.69, 723–731 (2017).
-
- Willems, R. P. et al. Incidence of infection with multidrug-resistant Gram-negative bacteria and vancomycin-resistant enterococci in carriers: A systematic review and meta-regression analysis. Lancet Infect. Dis. (2023). - PubMed
-
- Chernov, V. M., Chernova, O. A., Mouzykantov, A. A., Lopukhov, L. L. & Aminov, R. I. Omics of antimicrobials and antimicrobial resistance. Expert Opin. Drug Discov.14, 455–468 (2019). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

